Abstract
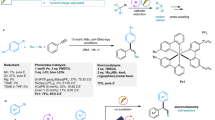
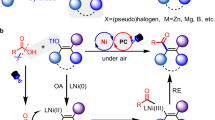
The development of general and efficient strategies for the construction of allenes is important due to their wide applications. Although few protocols have been developed via the 1,4-difunctionalization of 1,3-enynes under thermal or photoredox conditions, the mild and robust methodology for dicarbofunctionalization and hydroalkylation remains unexplored. In the present study, we report an electrochemical multicomponent protocol for the chemo- and regioselective difunctionalization of 1,3-enynes. In particular, 1,4-arylalkylation and unsymmetrical dialkylation have been realized via electro- and nickel dual catalysis using graphite/nickel foam and zinc/nickel foam as electrodes, respectively. The use of a Zn/reticulated vitreous carbon electrode led to efficient 1,4-hydro(deutero)alkylation in the absence of a metal catalyst. A wide range of structurally diverse tri- and tetra-substituted allenes were easily prepared with good efficiency and excellent regioselectivity under mild reaction conditions. Notably, a series of natural product- and drug-derived substrates could undergo late-stage functionalization to generate the corresponding complex allenes.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the data generated in the present study are available within the article and the Supplementary Information. The cartesian coordinates of DFT calculations are available in Supplementary Data 1.
References
Krause, N. & Hashmi, A. S. Modern Allene Chemistry (Wiley-VCH, 2004).
Hoffmann‐Röder, A. & Krause, N. Synthesis and properties of allenic natural products and pharmaceuticals. Angew. Chem. Int. Ed. 43, 1196–1216 (2004).
Rivera‐Fuentes, P. & Diederich, F. Allenes in molecular materials. Angew. Chem. Int. Ed. 51, 2818–2828 (2012).
Yu, S. & Ma, S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 51, 3074–3112 (2012).
Reich, H. J. & Willis, W. W. Jr Organoselenium chemistry. Formation of acetylenes and allenes by syn elimination of vinyl selenoxides. J. Am. Chem. Soc. 102, 5967–5968 (1980).
Liu, W. B., He, H., Dai, L. X. & You, S. L. A one‐pot palladium‐catalyzed allylic alkylation and Wittig reaction of phosphorus ylides. Chem. Eur. J. 16, 7376–7379 (2010).
Saget, T. & Cramer, N. Heteroatom‐nucleophile‐induced C-C fragmentations: synthesis of allenes and entry to domino reactions. Angew. Chem. Int. Ed. 49, 8962–8965 (2010).
Liu, H., Leow, D., Huang, K.-W. & Tan, C.-H. Enantioselective synthesis of chiral allenoates by guanidine-catalyzed isomerization of 3-alkynoates. J. Am. Chem. Soc. 131, 7212–7213 (2009).
Ito, H., Sasaki, Y. & Sawamura, M. Copper (I)-catalyzed substitution of propargylic carbonates with diboron: selective synthesis of multisubstituted allenylboronates. J. Am. Chem. Soc. 130, 15774–15775 (2008).
Coeffard, V., Aylward, M. & Guiry, P. J. First regio‐ and enantioselective chromium‐catalyzed homoallenylation of aldehydes. Angew. Chem. Int. Ed. 48, 9152–9155 (2009).
Lee, P. H., Lee, K. & Kang, Y. In situ generation of vinyl allenes and its applications to one-pot assembly of cyclohexene, cyclooctadiene, 3,7-nonadienone, and bicyclo[6.4.0]dodecene derivatives with palladium-catalyzed multicomponent reactions. J. Am. Chem. Soc. 128, 1139–1146 (2006).
Tietze, L. F. & Modi, A. Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 20, 304–322 (2000).
Masson, G., Neuville, L., Bughin, C., Fayol, A. & Zhu, J. in Synthesis of Heterocycles via Multicomponent Reactions II (eds Orru, R. V. A. & Ruijter, E.) 1–24 (Springer, 2010).
Domling, A., Wang, W. & Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012).
Zhu, J., Wang, Q. & Wang, M. Multicomponent Reactions in Organic Synthesis (John Wiley & Sons, 2014).
Levi, L. & Müller, T. J. Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 45, 2825–2846 (2016).
Apolinar, O. et al. Three-component asymmetric Ni-catalyzed 1,2-dicarbofunctionalization of unactivated alkenes via stereoselective migratory insertion. J. Am. Chem. Soc. 144, 19337–19343 (2022).
Tran, V. T. et al. Integrating allyl electrophiles into nickel‐catalyzed conjunctive cross‐coupling. Angew. Chem. Int. Ed. 59, 7029–7034 (2020).
Aryal, V. et al. Ni-catalyzed regio-and stereoselective alkylarylation of unactivated alkenes in γ,δ-alkenylketimines. ACS Catal. 12, 7262–7268 (2022).
Dhungana, R. K. et al. Ni‐catalyzed arylbenzylation of alkenylarenes: kinetic studies reveal autocatalysis by ZnX2. Angew. Chem. Int. Ed. 60, 22977–22982 (2021).
Campbell, M. W., Yuan, M., Polites, V. C., Gutierrez, O. & Molander, G. A. Photochemical C–H activation enables nickel-catalyzed olefin dicarbofunctionalization. J. Am. Chem. Soc. 143, 3901–3910 (2021).
Huang, W., Keess, S. & Molander, G. A. Dicarbofunctionalization of [1.1.1]propellane enabled by nickel/photoredox dual catalysis: one-step multicomponent strategy for the synthesis of BCP-aryl derivatives. J. Am. Chem. Soc. 144, 12961–12969 (2022).
Huang, W., Keess, S. & Molander, G. A. One step synthesis of unsymmetrical 1,3-disubstituted BCP ketones via nickel/photoredox-catalyzed [1.1.1]propellane multicomponent dicarbofunctionalization. Chem. Sci. 13, 11936–11942 (2022).
Long, T. et al. Ligand-controlled stereodivergent alkenylation of alkynes to access functionalized trans- and cis-1,3-dienes. Nat. Commun. 14, 55 (2023).
Li, X. et al. Three-component enantioselective alkenylation of organophosphonates via nickel metallaphotoredox catalysis. Chem 9, 154–169 (2023).
Zhu, C., Yue, H. & Rueping, M. Nickel catalyzed multicomponent stereodivergent synthesis of olefins enabled by electrochemistry, photocatalysis and photo-electrochemistry. Nat. Commun. 13, 3240 (2022).
Yue, H., Zhu, C., Kancherla, R., Liu, F. & Rueping, M. Regioselective hydroalkylation and arylalkylation of alkynes by photoredox/nickel dual catalysis: application and mechanism. Angew. Chem. Int. Ed. 59, 5738–5746 (2020).
Zhu, C. et al. A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer. Nat. Catal. 2, 678–687 (2019).
Chen, Y., Zhu, K., Huang, Q. & Lu, Y. Regiodivergent sulfonylarylation of 1,3-enynes via nickel/photoredox dual catalysis. Chem. Sci. 12, 13564–13571 (2021).
Wang, L., Ma, R., Sun, J., Zheng, G. & Zhang, Q. NHC and visible light-mediated photoredox co-catalyzed 1,4-sulfonylacylation of 1,3-enynes for tetrasubstituted allenyl ketones. Chem. Sci. 13, 3169–3175 (2022).
Song, Y., Fu, C. & Ma, S. Copper-catalyzed syntheses of multiple functionalizatized allenes via three-component reaction of enynes. ACS Catal. 11, 10007–10013 (2021).
Chen, Y., Wang, J. & Lu, Y. Decarboxylative 1,4-carbocyanation of 1,3-enynes to access tetra-substituted allenes via copper/photoredox dual catalysis. Chem. Sci. 12, 11316–11321 (2021).
Zhu, X. et al. Copper-catalyzed radical 1,4-difunctionalization of 1,3-enynes with alkyl diacyl peroxides and N-fluorobenzenesulfonimide. J. Am. Chem. Soc. 141, 548–559 (2018).
Zeng, Y. et al. Copper-catalyzed enantioselective radical 1,4-difunctionalization of 1,3-enynes. J. Am. Chem. Soc. 142, 18014–18021 (2020).
Dong, X. Y. et al. Copper‐catalyzed asymmetric coupling of allenyl radicals with terminal alkynes to access tetrasubstituted allenes. Angew. Chem. Int. Ed. 60, 2160–2164 (2021).
Wang, F. et al. Divergent synthesis of CF3‐substituted allenyl nitriles by ligand‐controlled radical 1,2‐ and 1,4‐addition to 1,3‐enynes. Angew. Chem. Int. Ed. 57, 7140–7145 (2018).
Terao, J., Bando, F. & Kambe, N. Ni-catalyzed regioselective three-component coupling of alkyl halides, arylalkynes, or enynes with R–M (M = MgX′, ZnX′). Chem. Commun. 47, 7336–7338 (2009).
Zhang, K. F. et al. Nickel‐catalyzed carbofluoroalkylation of 1,3‐enynes to access structurally diverse fluoroalkylated allenes. Angew. Chem. Int. Ed. 58, 5069–5074 (2019).
Ye, C. et al. Copper-catalyzed 1,4-alkylarylation of 1,3-enynes with masked alkyl electrophiles. Chem. Sci. 10, 3632–3636 (2019).
Ohira, Y., Mori, T., Hayashi, M., Onodera, G. & Kimura, M. Three-component coupling reaction of enynes, carbonyls, and organozinc reagents. Heterocycles 90, 832–841 (2015).
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Weix, D. J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015).
Gu, J., Wang, X., Xue, W. & Gong, H. Nickel-catalyzed reductive coupling of alkyl halides with other electrophiles: concept and mechanistic considerations. Org. Chem. Front. 2, 1411–1421 (2015).
Liu, J., Ye, Y., Sessler, J. L. & Gong, H. Cross-electrophile couplings of activated and sterically hindered halides and alcohol derivatives. Acc. Chem. Res. 53, 1833–1845 (2020).
Xue, W. et al. Nickel-catalyzed formation of quaternary carbon centers using tertiary alkyl electrophiles. Chem. Soc. Rev. 50, 4162–4184 (2021).
Liu, J., Lu, L., Wood, D. & Lin, S. New redox strategies in organic synthesis by means of electrochemistry and photochemistry. ACS Cent. Sci. 6, 1317–1340 (2020).
Gandeepan, P., Finger, L. H., Meyer, T. H. & Ackermann, L. 3D metallaelectrocatalysis for resource economical syntheses. Chem. Soc. Rev. 49, 4254–4272 (2020).
Ackermann, L. Metalla-electrocatalyzed C–H activation by earth-abundant 3d metals and beyond. Acc. Chem. Res. 53, 84–104 (2019).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature 535, 406–410 (2016).
Gao, X., Wang, P., Zeng, L., Tang, S. & Lei, A. Cobalt(II)-catalyzed electrooxidative C–H amination of arenes with alkylamines. J. Am. Chem. Soc. 140, 4195–4199 (2018).
Zhang, B. et al. Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling. Nature 606, 313–318 (2022).
Zhang, W. et al. Electrochemically driven cross-electrophile coupling of alkyl halides. Nature 604, 292–297 (2022).
Hamby, T. B., LaLama, M. J. & Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 376, 410–416 (2022).
Lu, L., Siu, J. C., Lai, Y. & Lin, S. An electroreductive approach to radical silylation via the activation of strong Si–Cl bond. J. Am. Chem. Soc. 142, 21272–21278 (2020).
Zhang, W. & Lin, S. Electroreductive carbofunctionalization of alkenes with alkyl bromides via a radical-polar crossover mechanism. J. Am. Chem. Soc. 142, 20661–20670 (2020).
Fu, N. et al. New bisoxazoline ligands enable enantioselective electrocatalytic cyanofunctionalization of vinylarenes. J. Am. Chem. Soc. 141, 14480–14485 (2019).
Siu, J. C. et al. Electrochemical azidooxygenation of alkenes mediated by a Tempo–N3 charge-transfer complex. J. Am. Chem. Soc. 140, 12511–12520 (2018).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Till, N. A., Oh, S., MacMillan, D. W. & Bird, M. J. The application of pulse radiolysis to the study of Ni(I) intermediates in Ni-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 143, 9332–9337 (2021).
Ting, S. I., Williams, W. L. & Doyle, A. G. Oxidative addition of aryl halides to a Ni(I)-bipyridine complex. J. Am. Chem. Soc. 144, 5575–5582 (2022).
Lin, Q. & Diao, T. Mechanism of Ni-catalyzed reductive 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 17937–17948 (2019).
Diccianni, J., Lin, Q. & Diao, T. Mechanisms of nickel-catalyzed coupling reactions and applications in alkene functionalization. Acc. Chem. Res. 53, 906–919 (2020).
Lin, Q., Fu, Y., Liu, P. & Diao, T. Monovalent nickel-mediated radical formation: a concerted halogen-atom dissociation pathway determined by electroanalytical studies. J. Am. Chem. Soc. 143, 14196–14206 (2021).
Chen, H., Yue, H., Zhu, C. & Rueping, M. Reactivity in nickel‐catalyzed multi‐component sequential reductive cross‐coupling reactions. Angew. Chem. Int. Ed. 61, e202204144 (2022).
Maity, B. et al. Mechanistic insight into the photoredox-nickel-HAT triple catalyzed arylation and alkylation of α-amino Csp3–H bonds. J. Am. Chem. Soc. 142, 16942–16952 (2020).
Acknowledgements
This work was financially supported by the King Abdullah University of Science and Technology (KAUST) and the Saudi Arabia, Office of Sponsored Research (URF/1/4025). C.Z. acknowledges the KAUST Supercomputing Laboratory for providing computational resources from the supercomputer Shaheen II (k1284).
Author information
Authors and Affiliations
Contributions
C.Z., H.Y. and M.R. conceived and designed the project. C.Z., H.C. and H.Y. optimized the reaction conditions. H.C. performed and analysed the experiments. C.Z. and H.C. performed the experimental mechanistic studies. C.Z. performed the theoretical calculations. C.Z., H.Y. and M.R. co-wrote the manuscript. M.R. directed the whole research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary sections 1–10, including experimental details, Figs. 1–6 and Tables 1 and 2.
Supplementary Data 1
The cartesian coordinates of DFT calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, C., Chen, H., Yue, H. et al. Electrochemical chemo- and regioselective arylalkylation, dialkylation and hydro(deutero)alkylation of 1,3-enynes. Nat. Synth 2, 1068–1081 (2023). https://doi.org/10.1038/s44160-023-00349-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00349-9
This article is cited by
-
Electroreduction of unactivated alkenes using water as hydrogen source
Nature Communications (2024)