Abstract
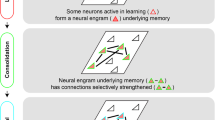
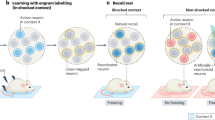
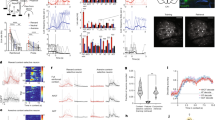
Memory retrieval involves the interaction between external sensory or internally generated cues and stored memory traces (or engrams) in a process termed ‘ecphory’. While ecphory has been examined in human cognitive neuroscience research, its neurobiological foundation is less understood. To the extent that ecphory involves ‘reawakening’ of engrams, leveraging recently developed technologies that can identify and manipulate engrams in rodents provides a fertile avenue for examining retrieval at the level of neuronal ensembles. Here we evaluate emerging neuroscientific research of this type, using cognitive theory as a guiding principle to organize and interpret initial findings. Our Review highlights the critical interaction between engrams and retrieval cues (environmental or artificial) for memory accessibility and retrieval success. These findings also highlight the intimate relationship between the mechanisms important in forming engrams and those important in their recovery, as captured in the cognitive notion of ‘encoding specificity’. Finally, we identify several questions that currently remain unanswered.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tulving, E. & Pearlstone, Z. Availability versus accessibility of information in memory for words. J. Verbal Learn. Verbal Behav. 5, 381–391 (1966).
Tulving, E. Ecphoric processes in episodic memory. Philos. Trans. R. Soc. Lond. B 302, 361–370 (1983).
Tulving, E. Elements of Episodic Memory. (Oxford University Press, 1983).
Semon, R. Die Mneme. (W. Engelmann, 1904).
Josselyn, S. A., Köhler, S. & Frankland, P. W. Heroes of the engram. J. Neurosci. 37, 4647–4657 (2017).
Tulving, E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 53, 1–25 (2002).
Eichenbaum, H. Still searching for the engram. Learn. Behav. 44, 209–222 (2016).
Josselyn, S. A., Köhler, S. & Frankland, P. W. Finding the engram. Nat. Rev. Neurosci. 16, 521–534 (2015).
Tonegawa, S., Liu, X., Ramirez, S. & Redondo, R. Memory engram cells have come of age. Neuron 87, 918–931 (2015).
Tonegawa, S., Pignatelli, M., Roy, D. S. & Ryan, T. J. Memory engram storage and retrieval. Curr. Opin. Neurobiol. 35, 101–109 (2015).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Denny, C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014).
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Sørensen, A. T. et al. A robust activity marking system for exploring active neuronal ensembles. eLife 5, e13918 (2016).
Josselyn, S. A. & Frankland, P. W. Memory allocation: mechanisms and function. Annu. Rev. Neurosci. 41, 389–413 (2018).
Ben-Yakov, A., Dudai, Y. & Mayford, M. R. Memory retrieval in mice and men. Cold Spring Harb. Perspect. Biol. 7, a021790 (2015).
Robins, S. K. Memory and optogenetic intervention: separating the engram from the ecphory. Philos. Sci. 85, 1078–1089 (2018).
Denny, C. A., Lebois, E. & Ramirez, S. From engrams to pathologies of the brain. Front. Neural Circuits 11, 23 (2017).
Tanaka, K. Z. et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354 (2014).
Han, J. H. et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009).
Rashid, A. J. et al. Competition between engrams influences fear memory formation and recall. Science 353, 383–387 (2016).
Zhou, Y. et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12, 1438–1443 (2009).
Sano, Y. et al. CREB regulates memory allocation in the insular cortex. Curr. Biol. 24, 2833–2837 (2014).
Hsiang, H. L. et al. Manipulating a “cocaine engram” in mice. J. Neurosci. 34, 14115–14127 (2014).
Lacagnina, A. F. et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci. 22, 753–761 (2019).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Yiu, A. P. et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83, 722–735 (2014).
Kim, J., Kwon, J. T., Kim, H. S., Josselyn, S. A. & Han, J. H. Memory recall and modifications by activating neurons with elevated CREB. Nat. Neurosci. 17, 65–72 (2014).
Abdou, K. et al. Synapse-specific representation of the identity of overlapping memory engrams. Science 360, 1227–1231 (2018).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014).
Cowansage, K. K. et al. Direct reactivation of a coherent neocortical memory of context. Neuron 84, 432–441 (2014).
Vetere, G. et al. Memory formation in the absence of experience. Nat. Neurosci. 22, 933–940 (2019).
Roy, D. S., Muralidhar, S., Smith, L. M. & Tonegawa, S. Silent memory engrams as the basis for retrograde amnesia. Proc. Natl. Acad. Sci. USA 114, E9972–E9979 (2017).
Nader, K., Schafe, G. E. & Le Doux, J. E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000).
Suzuki, A. et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795 (2004).
Roy, D. S. et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512 (2016).
Perusini, J. N. et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus 27, 1110–1122 (2017).
Guskjolen, A. et al. Recovery of “lost” infant memories in mice. Curr. Biol. 28, 2283–2290.e3 (2018).
Okuyama, T., Kitamura, T., Roy, D. S., Itohara, S. & Tonegawa, S. Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016).
Choi, J. H. et al. Interregional synaptic maps among engram cells underlie memory formation. Science 360, 430–435 (2018).
Gouty-Colomer, L. A. et al. Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry 21, 364–375 (2016).
Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014).
Schacter, D.L. Searching for Memory: The Brain, the Mind, and the Past. (Basic Books, 1996).
Roy, D. S., Okuyama, T. & Tonegawa, S. Tagging activated neurons with light. Nat. Biotechnol. 35, 827–828 (2017).
Davis, R. L. & Zhong, Y. The biology of forgetting—a perspective. Neuron 95, 490–503 (2017).
Frankland, P. W., Köhler, S. & Josselyn, S. A. Hippocampal neurogenesis and forgetting. Trends Neurosci. 36, 497–503 (2013).
Hardt, O., Nader, K. & Nadel, L. Decay happens: the role of active forgetting in memory. Trends Cogn. Sci. 17, 111–120 (2013).
Tulving, E. & Thomson, D. M. Encoding specificity and retrieval processes in episodic memory. Psychol. Rev. 80, 352–373 (1973).
Eich, E. Mood as a mediator of place dependent memory. J. Exp. Psychol. Gen. 124, 293–308 (1995).
Godden, D. R. & Baddeley, A. D. Context‐dependent memory in two natural environments: On land and underwater. Br. J. Psychol. 66, 325–331 (1975).
Smith, S. M. & Vela, E. Environmental context-dependent memory: a review and meta-analysis. Psychon. Bull. Rev. 8, 203–220 (2001).
Bouton, M. E. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 114, 80–99 (1993).
Maren, S., Phan, K. L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013).
Jafarpour, A., Fuentemilla, L., Horner, A. J., Penny, W. & Duzel, E. Replay of very early encoding representations during recollection. J. Neurosci. 34, 242–248 (2014).
Johnson, J. D., McDuff, S. G., Rugg, M. D. & Norman, K. A. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron 63, 697–708 (2009).
Manning, J. R., Polyn, S. M., Baltuch, G. H., Litt, B. & Kahana, M. J. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proc. Natl. Acad. Sci. USA 108, 12893–12897 (2011).
Polyn, S. M., Natu, V. S., Cohen, J. D. & Norman, K. A. Category-specific cortical activity precedes retrieval during memory search. Science 310, 1963–1966 (2005).
Ritchey, M., Wing, E. A., LaBar, K. S. & Cabeza, R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb. Cortex 23, 2818–2828 (2013).
Staresina, B. P., Henson, R. N., Kriegeskorte, N. & Alink, A. Episodic reinstatement in the medial temporal lobe. J. Neurosci. 32, 18150–18156 (2012).
Staresina, B. P. et al. Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. eLife 5, e17397 (2016).
Yaffe, R. B. et al. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proc. Natl. Acad. Sci. USA 111, 18727–18732 (2014).
St-Laurent, M., Abdi, H. & Buchsbaum, B. R. Distributed patterns of reactivation predict vividness of recollection. J. Cogn. Neurosci. 27, 2000–2018 (2015).
Danker, J. F., Tompary, A. & Davachi, L. Trial-by-trial hippocampal encoding activation predicts the fidelity of cortical reinstatement during subsequent retrieval. Cereb. Cortex 27, 3515–3524 (2017).
Horner, A. J., Bisby, J. A., Bush, D., Lin, W. J. & Burgess, N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun. 6, 7462 (2015).
Staresina, B. P., Cooper, E. & Henson, R. N. Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. J. Neurosci. 33, 14184–14192 (2013).
Staudigl, T., Vollmar, C., Noachtar, S. & Hanslmayr, S. Temporal-pattern similarity analysis reveals the beneficial and detrimental effects of context reinstatement on human memory. J. Neurosci. 35, 5373–5384 (2015).
Guzowski, J. F., McNaughton, B. L., Barnes, C. A. & Worley, P. F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124 (1999).
Deng, W., Mayford, M. & Gage, F. H. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2, e00312 (2013).
Khalaf, O. et al. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360, 1239–1242 (2018).
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013).
Tayler, K. K., Tanaka, K. Z., Reijmers, L. G. & Wiltgen, B. J. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 23, 99–106 (2013).
Richards, B. A. & Frankland, P. W. The conjunctive trace. Hippocampus 23, 207–212 (2013).
Orsini, C. A., Yan, C. & Maren, S. Ensemble coding of context-dependent fear memory in the amygdala. Front. Behav. Neurosci. 7, 199 (2013).
Emiliani, V., Cohen, A. E., Deisseroth, K. & Häusser, M. All-optical interrogation of neural circuits. J. Neurosci. 35, 13917–13926 (2015).
Yang, S. J. et al. Extended field-of-view and increased-signal 3D holographic illumination with time-division multiplexing. Opt. Express 23, 32573–32581 (2015).
Yang, W. & Yuste, R. Holographic imaging and photostimulation of neural activity. Curr. Opin. Neurobiol. 50, 211–221 (2018).
Carrillo-Reid, L., Han, S., Yang, W., Akrouh, A. & Yuste, R. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178, 447–457.e5 (2019).
Marshel, J. H. et al. Cortical layer-specific critical dynamics triggering perception. Science 365, eaaw5202 (2019).
Squire, L. R. & Alvarez, P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 5, 169–177 (1995).
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 100, 147–154 (1986).
McClelland, J. L., McNaughton, B. L. & O’Reilly, R. C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995).
Norman, K. A. & O’Reilly, R. C. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 110, 611–646 (2003).
Josselyn, S. A. & Frankland, P. W. Infantile amnesia: a neurogenic hypothesis. Learn. Mem. 19, 423–433 (2012).
Wheeler, A. L. et al. Identification of a functional connectome for long-term fear memory in mice. PLOS Comput. Biol. 9, e1002853 (2013).
Vetere, G. et al. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94, 363–374.e4 (2017).
Moscovitch, M., Cabeza, R., Winocur, G. & Nadel, L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67, 105–134 (2016).
Frankland, P. W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005).
Rescorla, R.A. Pavlovian Second-order Conditioning (Psychology Revivals): Studies in Associative Learning. (Psychology Press, 2014).
Ohkawa, N. et al. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep. 11, 261–269 (2015).
Chen, B. K. et al. Artificially enhancing and suppressing hippocampus-mediated memories. Curr. Biol. 29, 1885–1894.e4 (2019).
Anderson, M. C. Rethinking interference theory: Executive control and the mechanisms of forgetting. J. Mem. Lang. 49, 415–445 (2003).
Garner, A. R. et al. Generation of a synthetic memory trace. Science 335, 1513–1516 (2012).
Sekeres, M. J. et al. Recovering and preventing loss of detailed memory: differential rates of forgetting for detail types in episodic memory. Learn. Mem. 23, 72–82 (2016).
Richards, B. A. & Frankland, P. W. The persistence and transience of memory. Neuron 94, 1071–1084 (2017).
Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 7, 217–227 (1997).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017).
Tonegawa, S., Morrissey, M. D. & Kitamura, T. The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci. 19, 485–498 (2018).
Schapiro, A. C., Turk-Browne, N. B., Botvinick, M. M. & Norman, K. A. Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Philos. Trans. R. Soc. Lond. B 372, 20160049 (2017).
Cai, D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016).
Yokose, J. et al. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science 355, 398–403 (2017).
Gilboa, A. & Marlatte, H. Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci. 21, 618–631 (2017).
Richards, B. A. et al. Patterns across multiple memories are identified over time. Nat. Neurosci. 17, 981–986 (2014).
Tse, D. et al. Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895 (2011).
Barry, D. N. & Maguire, E. A. Remote memory and the hippocampus: a constructive critique. Trends Cogn. Sci. 23, 128–142 (2019).
Barry, D. N. & Maguire, E. A. Consolidating the case for transient hippocampal memory traces. Trends Cogn. Sci. 23, 635–636 (2019).
Moscovitch, M. & Nadel, L. Sculpting remote memory: enduring hippocampal traces and vmPFC reconstructive processes. Trends Cogn. Sci. 23, 634–635 (2019).
Han, J. H. et al. Neuronal competition and selection during memory formation. Science 316, 457–460 (2007).
Rogerson, T. et al. Molecular and cellular mechanisms for trapping and activating emotional memories. PLoS One 11, e0161655 (2016).
Kawashima, T. et al. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat. Methods 10, 889–895 (2013).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Acknowledgements
We thank A.Ramsaran and A.Park for drawing the figures, and we thank T. Ryan for comments on an earlier draft of this manuscript. This work was supported by Canadian Institutes of Health Research grants to P.W.F. (FDN-143227) and S.A.J. (FDN-388455) and a Natural Sciences and Engineering Research Council Discovery grant to S.K. (RGPIN-5770).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Stephen Maren and Steve Ramirez for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frankland, P.W., Josselyn, S.A. & Köhler, S. The neurobiological foundation of memory retrieval. Nat Neurosci 22, 1576–1585 (2019). https://doi.org/10.1038/s41593-019-0493-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0493-1
This article is cited by
-
Active forgetting and neuropsychiatric diseases
Molecular Psychiatry (2024)
-
Neurogenesis-dependent remodeling of hippocampal circuits reduces PTSD-like behaviors in adult mice
Molecular Psychiatry (2024)
-
Memory persistence: from fundamental mechanisms to translational opportunities
Translational Psychiatry (2024)
-
The cognitive impact of light: illuminating ipRGC circuit mechanisms
Nature Reviews Neuroscience (2024)
-
Perceived authenticity across three forms of educational simulations—the role of interactant representation, task alignment, and continuity of simulation
European Journal of Psychology of Education (2024)