Abstract
Tremendous strides have been made in our understanding of the neurobiological substrates of memory – the so-called memory “engram”. Here, we integrate recent progress in the engram field to illustrate how engram neurons transform across the “lifespan” of a memory — from initial memory encoding, to consolidation and retrieval, and ultimately to forgetting. To do so, we first describe how cell-intrinsic properties shape the initial emergence of the engram at memory encoding. Second, we highlight how these encoding neurons preferentially participate in synaptic- and systems-level consolidation of memory. Third, we describe how these changes during encoding and consolidation guide neural reactivation during retrieval, and facilitate memory recall. Fourth, we describe neurobiological mechanisms of forgetting, and how these mechanisms can counteract engram properties established during memory encoding, consolidation, and retrieval. Motivated by recent experimental results across these four sections, we conclude by proposing some conceptual extensions to the traditional view of the engram, including broadening the view of cell-type participation within engrams and across memory stages. In collection, our review synthesizes general principles of the engram across memory stages, and describes future avenues to further understand the dynamic engram.
Similar content being viewed by others
Introduction
Memory can be defined as an experience-dependent alteration in behavior that persists beyond the environmental stimuli that produced it. Memory is often conceptualized as a multi-staged process that includes encoding, consolidation, retrieval, and forgetting. As such, mechanistically interpreting memory in the brain is facilitated by understanding the neural underpinnings of each of these stages independently, as well as how these neural elements interrelate across stages. In this regard, significant progress has been made in our understanding memory stages at the level of ‘engram neurons’ – that is, neurons that mediate a particular memory across stages [1,2,3,4,5,6,7,8].
In this review, we seek to identify and connect key overarching principles — principles that seem to largely hold across neural regions and tasks — that lead to neurons participating across multiple memory stages and forming a cellular substrate of memory. Our review focuses primarily on rodent research from the hippocampus, amygdala, and medial prefrontal cortex (mPFC) [9], and is organized according to a typical order of memory stages: encoding, consolidation, retrieval, and forgetting (Fig. 1). We note that many excellent reviews on memory have been written on these memory stages (e.g. [1,2,3, 5, 8, 10,11,12]; and note other memory stages exist outside of this scope: e.g. [13,14,15]). Our goal here is to complement and build upon previous work by synthesizing understanding of engram neurons, both within and across these stages of memory. Motivated by recent empirical developments, we conclude our review by suggesting some important conceptual extensions as to how the engram is traditionally examined and understood.
Memory encoding
Intrinsic neuronal excitability regulates recruitment into the engram
The engram can be viewed as the physical change that occurred in the nervous system in response to a learned experience, which can later mediate instantiation of the corresponding memory. As such, engram neurons are typically defined as the neurons that are preferentially involved in the encoding, consolidation, and retrieval of a particular memory [8] (Box 1).
Why are some neurons, rather than their neighbors, recruited during the encoding of a memory? In principle, neurons might be preferentially recruited in memory encoding due to specialized intrinsic properties. Reinforcing this, intrinsic cellular excitability — the propensity of a neuron to fire an action potential in response to input — can be a key determinant of participation in memory [16]. In the context of memory, neurons with high excitability can be biased towards responding during learning and participating in memory encoding [16,17,18], illustrating in certain cases that intrinsic excitability can bias cells to being active during memory encoding.
To examine this principle from an interventional perspective, the transcription factor cAMP Response Element-Binding protein (CREB) is often leveraged as a tool to regulate neuronal activity during memory encoding [12, 19,20,21]. CREB regulates neuronal activity in a variety of subcortical and cortical regions [22,23,24,25,26], and across an array of memory tasks [26,27,28,29,30], and can thus regulate which neurons are allocated into the engram. Specifically, CREB-enhanced neurons are preferentially recruited into the engram, whereas CREB-deficient neurons are actively inhibited from encoding the memory [26, 31, 32]. Importantly, exogenous enhancement of CREB minutes before learning is sufficient to promote memory [33], thereby illustrating that CREB activity can shape memory on brief and behaviourally relevant timescales.
Such CREB-dependent recruitment is often posited to reflect changes in intrinsic neural excitability, with these changes in excitability shaping allocation during memory encoding. This posit has been empirically demonstrated in some cases, wherein selective suppression of the excitability of CREB-enhanced neurons prevents their preferential recruitment into the engram, whereas increasing excitability in a subset of neurons without manipulating levels of CREB enhances their recruitment into the engram [34]. This excitability-based mechanism of memory allocation is recapitulated under physiological conditions, with natural fluctuations in rates of neural excitability determining which neurons are selected to encode the corresponding memory [18]. It should be noted that the timescales of excitability plasticity changes can be faster than that of that CREB activity changes, and thus the extent to which natural fluctuations in CREB dictate allocation into the engram has yet to be demonstrated. Finally, it should also be noted that CREB shapes a host of disparate cellular functions, including synaptic plasticity [35]. Thus, CREB-driven neural activity may reflect changes beyond intrinsic properties, and its effects on intrinsic neuronal excitability may only account for some aspects of selection of neurons for memory encoding (Box 2). Ultimately, the extent to which endogenous CREB acts directly on excitability, and the CREB-dependent downstream cellular mechanisms that govern excitability, will be important avenues in future research.
The idea that intrinsic neuronal excitability plays an important role in memory allocation receives additional support from work in place cells [36, 37]. Activating place cells at a specific location promotes the formation of a place field corresponding to that location [38, 39] and targeted activation of place cells drives memory-guided spatial behavior [40], indicating that place cells form an essential neuronal underpinning of spatial memory. Most relevant to the current discussion, only a small subset of neurons become place cells during spatial learning, with most remaining silent [36, 41]. What dictates whether a neuron will become a place cell versus a non-responding silent cell? CA1 neurons that become place cells display higher rates of excitability from the beginning of exploration, and sometimes even before the animal is introduced to the new environment [42]. Remarkably, silent cells can be transformed into place cells with spatially tuned place fields by lowering their activation thresholds [43], potentially suggesting that increased intrinsic excitability may result in place cell emergence under physiological conditions. These data provide complementary CREB-independent support for the idea that the relative excitability of neurons at the time of learning helps determine which neurons will encode the corresponding memory, indicating that this may be a general principle of memory allocation.
In collection, while these findings converge on neural excitability regulating cellular recruitment during memory encoding, they do not negate that other factors influence memory encoding as well. Current evidence illustrates pre-existing patterns of synaptic connectivity (Fig. 1) and synaptic consolidation alongside neuromodulatory factors (Box 2) likely play essential roles here as well. Such mechanisms may emerge from, as well as be complemented by, epigenetic and other cell-intrinsic molecular properties that are engaged during learning and bias neurons towards an enduring role in memory [44,45,46,47]. Thus, while work on CREB and neuronal excitability has played (and will continue to play) a foundational role in our understanding of memory allocation, an important avenue for future research is uncovering complementary factors that predispose neurons to be an element of the engram.
Intrinsic neural excitability mediates formation of neuronal ensembles
Thus far, we have discussed the selection process determining which individual neurons are allocated into a memory. However, memory is often thought to be represented not at the level of individual neurons, but at the level of neuronal ensembles – that is, neuronal populations that show consistently synchronized activity in response to a particular stimulus, function, or mental state (e.g., a memory) [48]. Do neuronal ensembles effectively embody independent neurons, or are there specific interrelationships between neurons comprising an ensemble? Two-photon holographic optogenetics has been used to address this question in a highly specific manner [49,50,51]. Repetitive two-photon optogenetic activation of groups of neurons increases the probability of their firing together in the absence of external stimulus, consistent with the formation of a neuronal ensemble [50]. Fascinatingly, these neuronal ensembles are formed via cell-intrinsic upregulation of neural excitability between stimulated neurons, without a concomitant increase in synaptic plasticity (i.e., no new synaptic connections were made between previously unconnected neurons) [51]. These results suggest that memory formation occurs when highly excitable neurons display coordinated activity during memory encoding [4], and that the corresponding neuronal ensemble is formed based on levels of intrinsic excitability rather than alterations in synaptic plasticity per se (Box 2). While future work is needed to clarify these results (e.g., determining the extent to which they generalize across learning conditions), one interpretation is that highly excitable neurons increase the efficiency of already-existing synapses (Box 2). While these findings highlight the role of neuronal excitability in the selection and formation of neuronal ensembles, they do not negate the role of synaptic plasticity in the stabilization or consolidation of these ensembles once they are created. Indeed, in the time following the formation of a neuronal ensemble that underlies a memory, mechanisms of synaptic plasticity would be engaged to consolidate and strengthen this ensemble for future use.
Encoding summary
A competition-based rule can account for initial memory allocation, wherein excitable neurons and their associated neuronal ensembles out-compete less excitable counterparts for recruitment into the engram. Converging evidence for this rule has been obtained across an array of memory assays and neural regions. This rule therefore seems to be a generalizable feature of learning, and thus key for understanding the mechanisms underlying memory encoding.
Memory consolidation
Memory persistence requires synaptic consolidation
Recently encoded memories can be temporarily maintained via learning-induced increased activity [16, 51,52,53,54,55]. However, these memories are labile, highly susceptible to interference, and will rapidly decay without additional maintenance. The transformation of a short-term, labile memory into one that persists long-term requires gene expression and de novo protein synthesis. These processes culminate in increased synaptic coupling between active neurons co-active at the time of learning – a phenomenon called synaptic consolidation [56, 57]. Relatedly, manipulations that disrupt the molecular cascades involved in synaptic consolidation prevent memory consolidation [58, 59]. For example, through its influence on synaptic plasticity, suppressing CREB activity inhibits memory consolidation [57, 60]. Likewise, protein synthesis inhibitors prevent consolidation of memories if administered soon after learning [56, 57]. As such, synaptic consolidation represents a critical point of divergence, wherein consolidated memories survive and have the potential to be retrieved in the future, while those that aren’t targeted for synaptic consolidation may be lost (but see [61]).
Synaptic consolidation occurs preferentially in neurons active during learning
Neurons preferentially engaged during learning (i.e., putative engram neurons) selectively exhibit hallmark features of synaptic consolidation following memory encoding. For example, transcriptomic analysis has revealed a highly enriched CREB-dependent network that is recruited in engram neurons following contextual fear conditioning [62]. This CREB-dependent transcription promotes structural and functional changes preferentially in engram neurons, and is required for synaptic consolidation [62] (see Box 2). For example, GluR1 AMPA-Rs are preferentially expressed in dendritic spines of active CA1 neurons following contextual fear conditioning [63], and dentate gyrus engram neurons display increased spine density and synaptic strength following contextual fear conditioning [61]. Protein synthesis inhibitors administered immediately after learning abolish these engram-selective changes and culminate in failed memory consolidation [61]. Additionally, synaptic potentiation and the number and size of dendritic spines is selectively increased in engram-to-engram CA3-to-CA1 synapses following formation of a contextual fear memory [64]. Taken together, these results suggest that synaptic consolidation at the molecular, structural, and functional level occur selectively in the neurons active in response to a learning experience.
Neuronal reactivation drives early consolidation
The probability of forming a long-term hippocampal-dependent memory increases upon repeated behavioural exposures to the learning event, and intriguingly, repeated internal representations of the learning event also occur during behaviourally ‘offline’ periods. During these offline times, such as sleep or quiet wakefulness, patterns of activity among recently active hippocampal neurons is spontaneously replayed. Such replay events occur in either a forward or backward direction [65, 66] in a temporally-compressed format – upwards of 20x faster than occurred during the initial learning experience [67, 68]. Hippocampal replay events occur selectively during sharp wave ripples (a form of high frequency network oscillation) and drive memory consolidation [67, 69,70,71,72,73,74,75,76]. For example, optogenetic increase of sharp wave ripple duration improves consolidation of the corresponding memory [73], and selective disruption of replay prevents consolidation of the corresponding memory [77]. Furthermore, memory replay doesn’t simply reflect the strongest representation rising to the surface, but often occurs for memories most in need of consolidation (i.e., those most at risk degradation) [78, 79]. In accordance with this, targeted reactivation of fear-conditioning-induced lateral amygdala engram neurons during consolidation increases subsequent memory strength [80] (for conceptually similar results in the retrosplenial cortex, see [81]). Moreover, these fear memory engram neurons are preferentially reactivated during sleep, and optogenetically inhibiting their reactivation during sleep (but not later waking periods) prevents memory consolidation [82,83,84]. These findings converge on the idea that internally generated replay strengthens recently formed memories.
Hippocampal-dependent memories undergo systems-level consolidation
In the days, weeks, and months (and potentially years, in humans) following synaptic consolidation, the initially hippocampal-dependent component of memory undergoes extreme reorganization and redistribution such that it can be stored and expressed in a hippocampal-independent, mPFC-dependent format. This spatial reorganization of memory is known as systems consolidation. While hippocampal and mPFC-neocortical ensembles representing the same experience can co-exist in the brain [85,86,87], the phenomenological (or subjective) qualities of the memory depend on which neuronal ensemble is activated. Hippocampal-dependent memories are context-specific and detailed (i.e., episodic), whereas mPFC-dependent memories are associated with a more gist-like quality [87,88,89,90,91,92]. This is especially true following systems consolidation, after which mPFC ensembles come to represent commonalities among individual experience to generate a more schematized or generalized representation [93,94,95]. While time since encoding plays an important role in determining whether the hippocampal or neocortical component of the memory will be expressed during memory retrieval (in accordance with standard consolidation theory [96] and results discussed below), other factors such as task demands, attention, and prior knowledge are important factors as well [87].
Hippocampal engram reactivation drives systems consolidation
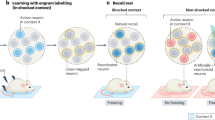
In contrast to hippocampal-dependent memories that consolidate within hours, mPFC-dependent memories generally take weeks to consolidate and contribute to memory storage and retrieval [97]. Structurally, mPFC engram neurons take multiple weeks to display learning-mediated increases in dendritic spine density [97], as well as increased synapse-specific strengthening between mPFC engram neurons [98]. What mechanisms promote maturation of mPFC-dependent memory and subsequent completion of systems consolidation? It has long been hypothesized that periods of offline hippocampal activity promote systems consolidation in cortical regions [36, 99]. One parsimonious framework for understanding the role of hippocampus in driving consolidation of a mPFC-dependent memory is indexing theory. According to this idea, the hippocampus forms an index (i.e., a pointer or address book) of the pattern of neocortical activity that was present during initial memory encoding, such that its activation promotes neocortical reinstatement (Fig. 2; Box 3).
Schematic of indexing theory across stages. During initial learning (red stage), communication between an index region (left, akin to the hippocampus) and a perceptual region (right, akin to the neocortex) results in coordinated activity between two ensembles (triangular cells and circular cells, respectively). Consolidation (green stage) strengthens this connectivity between ensembles. Later exposure to a partial contextual cue (gold stage) incompletely activates the perceptual ensemble, but this is sufficient to drive the entire index population. This drives retrieval (teal stage), wherein complete activation of the index cells is sufficient to drive activity across the full perceptual ensemble. At each stage, arrows illustrate flow of information.
One intuitive prediction of the hippocampal indexing theory is that hippocampal activity is required for the maturation and consolidation of mPFC-dependent memory. In line with this prediction, preventing hippocampal engram activity throughout systems consolidation prevents maturation of the mPFC engram, such that these neurons no longer display learning-mediated increases in dendritic spine density, increased engram-to-engram synaptic connectivity, or reactivation during memory retrieval [97, 98, 100] (for conceptually similar results, see [101, 102]). Similarly, place cells in the mPFC require hippocampal activity to form but not to persist [101], and suppressing post-learning hippocampal sharp wave ripples and replay impairs systems memory consolidation [103]. Hippocampal sharp wave ripples become coupled with mPFC spindles (low frequency oscillatory events) following contextual fear conditioning [104], and impairing spindle-ripple coupling post-training prevents consolidation of both recent and remote memory [104]. This result is important, as it suggests that memory replay must be coordinated between the hippocampus and mPFC for memory consolidation to succeed [104]. Reinforcing this, increased hippocampal-mPFC ripple-spindle coupling only occurs when learning results in successful consolidation [105]. Through repeated bouts of hippocampal-to-mPFC replay during sleep, the neocortical memory will stabilize and can eventually be supported independent of the hippocampus, thereby concluding systems consolidation [69,70,71].
Consolidation summary
The transformation of a short-term, labile memory into one that persists long-term requires synaptic and systems consolidation – a process that occurs preferentially in neurons active during memory encoding. Such processes take engram neurons involved in the encoding of memory, and promote connections of both local and long-range ensembles. Such spatially and temporally dynamic processing enables memory persistence via evolving activity that can embody distinct aspects of memory.
Memory Retrieval
How we remember: the encoding specificity principle
Once an engram has been consolidated and stored, it can be activated to induce memory retrieval [5]. What dictates successful memory retrieval? According to the encoding specificity principle, memory retrieval success is dictated by the extent to which the context (or cues) at retrieval matches that which was present during encoding [106]. The encoding specificity principle can be broken down into the principles of context-dependent memory and state-dependent memory, which deal with how well the external context and internal neurophysiological state of the animal match between encoding and retrieval, respectfully. The principle of encoding specificity is well established and has been documented in both human and non-human animals using an array of external environments and internal states [107,108,109,110].
Memory retrieval is associated with reactivation of neurons recruited during learning
From the encoding specificity principle, it follows that memory retrieval will be successful to the extent that the brain recapitulates patterns of neural activity that were present during memory encoding. The first evidence of retrieval-induced reinstatement of a putative engram was found by leveraging the time-dependent shift in the location of Arc RNA to identify the activation history of individual neurons at two different timepoints (i.e., catFISH). Through this technique, it was found that retrieval of a context memory preferentially reactivates putative CA1 engram neurons that were active during memory encoding [111]. This result was followed up by work using transgenic (TetTag) mice that allowed for the persistent tagging of active (c-Fos+) neurons in a narrow time window [112]. Using this system, it was found that fear memory retrieval increased reinstatement of tagged BLA engram neurons, with rates of engram reactivation predicting memory strength [112]. These results provided the first cellular-level observation that memory retrieval is associated with reengagement of the neuronal ensemble that encoded the memory and set the stage for much of what the ‘engram field’ has become today. That memory retrieval is associated with reactivation of neurons that were engaged during learning has been replicated using a variety of engram tagging techniques, in many different memory paradigms, and across an array of neural regions (for review, see [5, 8]).
One inherent challenge of many engram studies is that the experimental context animals are exposed to during memory retrieval is effectively identical to that which they experienced during learning. This experimental design can, in principle, make it difficult to tease apart whether engram reactivation reflects memory retrieval per se, as opposed to these neurons simply becoming active in response to particular features of the environment. Here, it is important to note that natural engram reactivation scales with strength of memory retrieval following fear extinction [13, 98, 112], natural forgetting [113], and in pathophysiological states characterized by memory impairments [13, 114, 115]. That engram reactivation scales with success of memory retrieval provides compelling evidence for a bone fide role in memory retrieval, as opposed to simply being evoked by a particular set of stimuli during a memory test.
Silencing engram neurons disrupts memory retrieval
If the neurons active in response to learning form a critical and enduring component of the memory, then selectively silencing these neurons should disrupt retrieval of the corresponding memory. In a series of influential studies, pre-training neural excitability was amplified in a subset of lateral amygdala or hippocampal neurons, thereby directing a targeted memory into these neurons. Targeted ablation [31] or inhibition [27, 29, 34, 116] of these engram neurons selectively disrupted retrieval of the corresponding memory (without disrupting the ability to learn new information), illustrating that the neural ensemble that encodes memory plays an enduring and necessary role in mediating memory retrieval. Converging evidence for this conclusion have been obtained across an array of neural regions (e.g., dentate gyrus and CA3 [117], CA1 [100], insular cortex [30], nucleus accumbens [118], mPFC [97, 119]) and therefore seems to be a generalizable feature of memory retrieval.
Activating engram neurons promotes memory retrieval
The encoding specificity principle, and in particular state-dependent memory, suggests that reinstating the state of the brain that was present during memory encoding should promote retrieval of the corresponding memory. While acknowledging the possibility of artificially inducing memory retrieval through direct engram reactivation, most assumed that the spatial-temporal firing patterns underlying memory retrieval were much too precise to be recapitulated via currently available neuron stimulation technology. Indeed, to many (perhaps most) in the field, the possibility of inducing memory retrieval through optogenetic or chemogenetic reactivation of the engram seemed about as likely as recreating Michelangelo’s David with a jackhammer instead of a chisel – our tools were simply too blunt and imprecise. Yet, to the surprise of almost everybody in the field, optogenetically or chemogenetically reactivating ‘memory encoding’ engram neurons induces (partial) retrieval of the corresponding memory, even in the absence of proper environmental retrieval cues [61, 120,121,122]. Simultaneous activation of engram neurons across multiple brain regions promotes stronger memory retrieval than activation of engram neurons within a single neuron region [86]. That memory retrieval can be induced by activating engram neurons has been widely replicated across neural regions [3, 5, 8] and behavioural paradigms (e.g., fear conditioning [61, 121]; conditioned place avoidance [123] and preference [118], go/no-go licking [49], inhibitory avoidance [114], object location memory [114], social preference memory [124]).
Memory retrieval can promote a transient increase in engram excitability that causally drives improved memory performance [125]. In this work involving contextual discrimination, memory retrieval promotes excitability for hours in dentate gyrus engram neurons. During the period of elevated engram excitability, animals display improved memory flexibility and accuracy in terms of pattern separation and completion at the behavioral level. Mechanistically, this retrieval-induced increase in engram excitability and the corresponding improvement in memory performance is cell-intrinsic, driven by changes in the inwardly rectifying potassium channel Kir2.1. Through its comprehensive measurements and direct manipulations of neuronal excitability, this study helps set the standard for casual relationships between engram excitability and memory processing [125].
To uncover robust effects related to memory, most studies in the field seek to manipulate large numbers of engram neurons. In this context, it is striking that optogenetic stimulation of as few as two visual cortex engram neurons is sufficient in driving pattern completion of the neuronal ensemble to which the neurons belong and retrieval of the corresponding memory [49]. Similarly, activation of hippocampal engram neurons promotes the reactivation of (non-stimulated) engram neurons in the amygdala and throughout the cortex [100, 126] (for an indexing theory interpretation of these results, see Box 3). Thus, while the stimulation protocols used in engram activation experiments are focal and largely non-physiological, their effects on the brain are widespread and recapitulate natural patterns of neuronal activity in areas downstream of the stimulated region of interest. The brain’s ability to complete patterns of activity (e.g., brain-wide engram) from incomplete input (e.g., dentate gyrus engram stimulation) likely explains the ability of focal stimulation of engram neurons to drive memory retrieval (Box 4).
Retrieval summary
According to the encoding specificity principle, memory retrieval success is dictated by the extent to which cues present at retrieval match that those present during encoding. Consistent with this idea, observational experiments illustrate memory retrieval is associated with reactivation of engram neurons engaged during initial learning. Interventionally, silencing neurons that were active during encoding suppresses memory retrieval, whereas activating these neurons promotes memory retrieval. These results hold across an array of tasks and neural regions, suggesting that the engram that is formed during encoding and strengthened during consolidation can be correlatively and causally linked to memory retrieval.
Forgetting
Forgetting as an adaptive phenomenon
Forgetting is often viewed as the lack of behavioural expression of a memory, which could be otherwise successfully recalled and expressed on an earlier occasion [11, 127]. According to this perspective, forgetting can occur because the memory is no longer available (i.e., complete engram degradation; a storage deficit) or because it is not currently accessible (i.e., a retrieval deficit). We note that our operational definition of forgetting here, which can be explained by both storage and retrieval failure, is assayed by behaviour and is agnostic to its biological cause. This varies from some stricter theoretical treatments wherein forgetting requires loss of memory representation per se.
Why do we forget? Memories are perhaps best understood as models of the future [94, 128], and once a memory no longer services predicting what the future might be like, it is best forgotten [129]. While forgetting can occur in a passive manner, for example in response to interfering environmental stimuli, it can also be a well-regulated and active process [130]. Indeed, it has been argued that the natural tendency of neural systems is to degrade rather than preserve information [130, 131]. While forgetting has negative connotations, it is an adaptive phenomenon that promotes future mnemonic processing, decision making, emotional regulation, and mental health [94, 132, 133].
Synaptic remodeling: A general principle of forgetting
Synaptic plasticity is required for successful learning, but comes at the cost of potentially degrading information already stored in the circuit (i.e. the plasticity-stability dilemma [134]). Given sufficient synaptic remodeling of an engram-specific neuronal ensemble, forgetting is unavoidable: there is an inevitable tipping point beyond which information stored in the ensemble will be lost (i.e., unless another set of synapses takes over the memory representation [135, 136]). This conclusion follows naturally from the encoding specificity principle, and in particular state-dependent memory: as connectivity changes accumulate in an ensemble, the probability of faithfully recapitulating the pattern of activity that underlie memory retrieval within that ensemble diminishes. In this way, synaptic remodeling of engram circuitry represents a general principle of how forgetting occurs in the brain (for review, see [137]).
Neurogenesis-mediated synaptic remodeling and forgetting
Any significant alteration to the synaptic connectivity within which an engram is embedded should lead to forgetting of the corresponding memory. Post-learning hippocampal neurogenesis is a powerful means through which hippocampal circuitry is remodeled and altered: as new dentate gyrus granule neurons mature, they infiltrate and reconfigure surrounding circuitry by forming connections with both presynaptic and post-synaptic partners [11]. As newborn neurons integrate into these pre-established circuits, their synaptic connections exist alongside and, in some cases, replace established synaptic connections [138,139,140]. In keeping with their capacity to remodel surrounding neural circuitry, post-learning hippocampal neurogenesis reduces engram reinstatement in downstream CA3 and CA1 [141] and promotes forgetting of the corresponding hippocampal-dependent memory [142,143,144,145,146]. Suppressing the extent to which adult-generated neurons structurally remodel hippocampal circuits (i.e., suppressing their addition of dendritic spines and mossy fiber terminals) prevents neurogenesis-mediated forgetting, whereas increasing the extent to which adult-generated dentate gyrus neurons remodel surrounding circuitry promotes forgetting, even without increasing overall rates of neurogenesis [141]. Thus, neurogenesis promotes forgetting by reconfiguring the circuitry within which hippocampal memories are embedded, thereby decreasing the probability of engram reactivation.
Microglial and astrocytic regulation of synaptic connectivity and forgetting
Microglia are the brain’s resident macrophage and immune cells. Interestingly, these cells also regulate synapse dynamics and thereby modulate rates of forgetting [147, 148]. Specifically, microglia-mediated synapse removal both decreases dentate gyrus engram reactivation and promotes forgetting of hippocampal-dependent memories [147, 149]. Interestingly, suppressing microglia-mediated synapse elimination also prevents neurogenesis-induced forgetting [147], thereby providing further evidence that neurogenesis induces forgetting via synaptic remodeling of hippocampal circuitry [11]. Astrocytes also regulate synapse dynamics via activity-dependent elimination of excitatory synapses, resulting in forgetting [150]. Interestingly, astrocyte activation can induce NMDA-dependent LTD via postsynaptic GluA2 AMPAR endocytosis [151], suggesting another mechanism through which astrocytes can promote forgetting (see discussion below). Thus, both removal of engram-related synapses [147, 149] and addition of redundant synapses [150] (i.e., bidirectional synaptic remodeling) promotes forgetting of hippocampal-dependent memories.
NMDA-R and AMPA-R mediated synaptic remodeling and forgetting
Synaptic depotentiation in response to ongoing neural activity (e.g., ordinary mental exertion) is considered one of the main causes of forgetting [152]. Consistent with this, preventing NMDA-mediated synaptic activity (particularly GluN2B-containing NMDA-R activity [153, 154]) blocks synaptic decay and concomitantly prevents forgetting of recently encoded hippocampal memories [153,154,155]. Similarly, the insertion and stabilization of GluA2-containing AMPA-Rs into post-synaptic sites is associated with synaptic strengthening and memory persistence, and NMDA-dependent removal of these GluA2 AMPA-Rs promotes synaptic depression and forgetting [151, 156,157,158,159,160]. Together, these results suggest that NMDA-R activity and GluA2 AMPA-R endocytosis disassemble the synaptic architecture that was put in place during consolidation; preventing these processes helps preserve the patterns of engram-to-engram synaptic connectivity that underlying the memory, thereby preventing forgetting [130, 159].
Intracellular signaling: Rac1-mediated regulating of synapse dynamics and forgetting
Intracellular signaling via the Rac1 (a small Rho GTPase) pathway regulates rates of forgetting in flies [161,162,163], mice [164, 165], and humans [161, 166]. Through its interaction with cofilin (a major promotor of actin cytoskeletal dynamics) [167], Rac1 regulates synapse structure and function and regulates both natural forgetting [164] and forgetting induced by disease states [161]. Whereas decreasing Rac1 activity in the hippocampus prevents synaptic decay and promotes memory persistence, increasing Rac1 availability accelerates synaptic decay and leads to earlier forgetting [164, 165]. Indeed, many of the mechanisms of forgetting already discussed converge on the Rac1 pathway. For example Rac1 activation in dentate gyrus engram neurons promotes microglia-induced synapse elimination and the forgetting of hippocampal-dependent memories [149]. Rac1 also regulates forgetting induced by neurogenesis-mediated circuit remodeling [141] and has been implicated in both NMDAR- and AMPAR-mediated forgetting [168]. Rac1 has also been leveraged to completely remove potentiated engram synapses, resulting in forgetting of the corresponding memory [169]. Together, these results indicate that loss of engram-specific synapses diminishes access to the information stored in that circuit (i.e., forgetting) [170,171,172] and highlights Rac1 as a key player in this process.
Neurophysiological noise-induced forgetting: engram reactivation without remembering
Neurophysiological noise that co-occurs during a memory retrieval attempt decreases signal-to-noise ratio and promotes forgetting [173]. For example, altering patterns of synaptic weights via LTP induction in hippocampal synapses promotes forgetting of hippocampal-dependent spatial memories [173]. Moreover, optogenetic or chemogenetic activation of non-engram neurons during memory retrieval promotes forgetting of memories dependent on that circuitry [49, 174,175,176]. In a somewhat counter-intuitive finding, neurophysiological noise at retrieval often promotes forgetting without decreasing rates of engram neuron reactivation [174, 175](but see [49]). Thus, whereas synaptic remodeling promotes forgetting by decreasing the probability of engram reactivation, neurophysiological noise can interfere with memory retrieval without preventing engram activation. The precise nature of this interference is unknown but likely involves a reduction in memory-related information flow between neural regions. More generally, these results suggest that potentiating synapses that are independent of the engram decreases signal-to-noise ratio, interferes with memory retrieval, and culminates in forgetting.
Role of engram availability and accessibility in forgetting
The results outlined above suggest that forgetting can occur because the memory is no longer available (i.e., engram degradation; a storage deficit) or because it is not currently accessible (i.e., a retrieval failure) [177]. Memories are often retrievable in situations where one might classically assume that the engram has degraded to the point where it is no longer available [61]. For example, memories ‘lost’ to infantile amnesia [126, 178] (for related work on infantile amnesia, see [179,180,181]) and neurogenesis-induced forgetting [141] can be recovered by optogenetic or chemogenetic stimulation of the dentate gyrus engram. Likewise, forgetting in transgenic mouse models of Alzheimer’s disease can be reversed via dentate gyrus engram stimulation [114, 115]. Work that has examined both memory recovery and spine dynamics has found that synaptic strength and spine density can be reduced to baseline levels in hippocampal engram neurons, but nonetheless memory can be recovered via dentate gyrus engram stimulation [61]. Relatedly, selective optogenetic-induced depression of engram synapses induces forgetting, whereas potentiation of these synapses reinstates the memory [170,171,172, 182].
These results indicate that forgetting is often the result of failed memory retrieval, as opposed to memory erasure. What is the neurobiological explanation for the survival of memory after such drastic synaptic rearrangements and loss of synaptic strength? There are at least three ways of explaining these data. One, some memory-associated synapses remain, and these spared synapses (whether within the targeted neural circuit or in downstream neural regions) are sufficient in storing the memory but not in driving memory retrieval behavior under physiological conditions. Two, the loss of engram-specific synaptic strength diminishes access to information stored in the circuit, but the information stored in the circuit can survive this loss of synaptic strength via persistence in engram-specific synaptic connectivity. According to this explanation, there is a critical distinction between the synaptic strength required for memory retrieval, and the synaptic connectivity required for memory storage (for further discussion and elaboration, see [3, 6, 61, 183, 184]). Three, while highly speculative, it remains possible that non-synaptic mechanisms may be capable of long-term memory storage [3, 47, 185, 186] (Box 5).
Forgetting summary
As synaptic changes in engram circuitry accumulate, so too does the probability of forgetting. In this way, synaptic remodeling of engram circuitry represents a general mechanism of forgetting. Such synaptic remodeling can occur from a variety of sources, including depotentiation of existing synapses, new synapses driven by ongoing neurogenesis, and synaptic elimination by non-neuronal cells. By disrupting the properties of engram synapses strengthened during early memory stages, circuit remodeling decreases the probability of engram reactivation and promotes forgetting. Nonetheless, engram stimulation experiments can evoke memory retrieval under certain conditions, illustrating that such remodeling does not necessarily produce complete memory erasure per se.
Future Considerations
Conceptual underpinnings and extensions of the engram
Engram cells are often conceived as neurons that (a) are active during initial learning, (b) display some persistent physical change in response to learning, and (c) are reactivated during (and required for) memory retrieval [1, 2, 8]. According to this strict definition, no new neurons are added or removed from the engram after it is formed, since these neurons did not participate in both encoding and retrieval (although we note that some definitions incorporate dynamicism; e.g. [7]). Viewed from this perspective, the engram is perceived as a relatively rigid and unchanging neurobiological entity – a fact that seems at odds with the inherently dynamic and constructive nature of memory. Considering this fact, we highlight a few key generalizations regarding the nature of engram cells, motivated by recent experimental progress: (1) non-neuronal engram cells exist, (2) actively inhibited neurons can be an essential component of the engram, (3) different engram neurons can contribute to different stages in memory processing, (4) determining the essential differences (as opposed to only the commonalities) between encoding and retrieval engrams is important to advance the field (i.e., encoding ensemble reactivation is an incomplete model of memory retrieval).
-
(1)
Existence of non-neuronal engram cells. Generally, the engram field places a heavy, almost exclusive emphasis on neuronal engram cells. However, it is highly likely that non-neuronal engrams exist, with astrocytes being a prime candidate. For example, emerging evidence suggests that astrocytes play an active role in information process, including regulating synaptic function, circuit connectivity, and memory retrieval [150, 187]. In addition, activation of astrocytes during memory encoding improves memory retrieval without altering basal synaptic transmission [188]. Similarly, the location of a mouse in a familiar maze can be predicted from astrocyte activity alone, suggesting that these cell types might directly encode spatial information [189]. The extent to which astrocytes are instructive engram cells (in addition to being permissive supporting cells) is worthy of serious consideration and experimentation.
-
(2)
Neural inactivity does not imply mnemonic passivity. A complete neurobiological understanding of the engram will include not just active neurons, but also actively inhibited neurons. Such active inhibition is often necessary for memory. As one example, the anterodorsal thalamic nucleus is necessary for the retrieval of recent, but not remote, contextual fear memory [190]. Notably, the anterodorsal thalamic nucleus needs to be actively inhibited at remote timepoints for memory retrieval to succeed [190]. Because this inhibition is necessary for content-specific memory retrieval success, these inhibited neurons ought to be considered a genuine component of the engram. Similarly, much as re-activating neurons that were active during encoding promotes memory retrieval, re-inhibiting neurons that were actively inhibited during encoding can also promote memory retrieval [191]. That such neurons are re-inhibited (rather than re-activated) during memory retrieval does not preclude them from being an essential component of the engram (for conceptually related work on inhibitory engrams, see [192, 193]).
-
(3)
Different neurons often underlie different stages of memory processing. Engram neurons are typically defined as neurons that were active during both encoding and retrieval of memory. Emerging evidence has illustrated, however, that some neurons play a critical role in memory encoding but do not have a similarly critical role in memory retrieval [194,195,196]. Conversely, there are neurons that play no clear role in memory encoding, but are recruited into the engram later during consolidation and contribute significantly to memory retrieval [98, 119, 194]. As such, a less strict definition of the engram may serve to help to amalgamate these complementary roles, incorporating ‘encoding engram neurons’ (i.e., neurons that are essential for memory encoding only), ‘retrieval engram neurons’ (i.e., neurons that are essential for memory retrieval only), and ‘reactivated engram neurons’ (i.e., neurons essential to both encoding and retrieval). Such terminology more accurately captures the dynamic nature of memory [7, 197, 198], and better highlights the role different engram neurons play in different stages of memory processing. Conceptually reframing engram neurons in this way could result in new and important research questions. As examples, what are the mechanisms and environmental factors that mediate the recruitment of new neurons into a pre-existing engram? What information is carried by neurons that participate in either encoding or retrieval, but not both?
-
(4)
‘Encoding’ engram reactivation is an incomplete model of memory retrieval. Memory is an inherently constructive process. In keeping with this, perception of an experience and memory retrieval of that experience are fundamentally distinct phenomena, with distinct psychological properties, and which must therefore engage – at least in part – distinct neural circuitry [199]. The engram field (including the current article) focuses almost exclusively on the commonalities between engram activation at encoding vs retrieval (i.e., engram reactivation) – and this remains a topic worthy of intensive study. However, it is equally important to study and understand the neurobiological differences between neural activity during memory encoding vs retrieval. Rather than being interpreted exclusively as noise or mnemonic imprecision, these differences in engram (in)activity could represent important (and adaptive) differences in how the brain processes perceptual information during encoding vs mnemonic information during retrieval.
Concluding remarks
The engram field, driven by new technology in combination with clever experimental design, has had a truly remarkable rate of recent discovery. The field is now able to visualize, measure, and manipulate engram neurons with an impressive level of specificity, enabling the role and evolution of the engram to be understood across memory stages. Such research has paved the way for exciting future opportunities to understand the engram across memory stages, in both traditional and non-canonical ways, and reveal the logic of memory in the brain.
References
Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Neuron. 2015;87:918–31.
Tonegawa S, Morrissey MD, Kitamura T. The role of engram cells in the systems consolidation of memory. Nat Rev Neurosci. 2018;19:485–98.
Tonegawa S, Pignatelli M, Roy DS, Ryan TJ. Memory engram storage and retrieval. Curr Opin Neurobiol. 2015;35:101–9.
Vetere G, Tran LM, Moberg S, Steadman PE, Restivo L, Morrison FG, et al. Memory formation in the absence of experience. Nat Neurosci. 2019;22:933–40.
Frankland PW, Josselyn SA, Köhler S. The neurobiological foundation of memory retrieval. Nat Neurosci. 2019;22:1576–85.
Ryan TJ, de San Luis CO, Pezzoli M, Sen S. Engram cell connectivity: an evolving substrate for information storage. Curr Opin Neurobiol. 2021;67:215–27.
Josselyn SA, Köhler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16:521–34.
Josselyn SA, Tonegawa S. Memory engrams: Recalling the past and imagining the future. Science. 2020;367:eaaw4325.
Laubach M, Amarante LM, Swanson K, White SR. What, if anything, is rodent prefrontal cortex? eneuro. 2018;5:ENEURO.0315–18.2018.
Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–86.
Frankland PW, Köhler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends Neurosci. 2013;36:497–503.
Josselyn SA, Frankland PW. Memory allocation: mechanisms and function. Ann Rev Neurosci. 2018;41:389–413.
Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci. 2019;22:753–61.
Zhang X, Kim J, Tonegawa S. Amygdala reward neurons form and store fear extinction memory. Neuron. 2020;105:1077–93. e7.
Cincotta C, Murawski NJ, Grella SL, McKissick O, Doucette E, Ramirez S. Chronic activation of fear engrams induces extinction‐like behavior in ethanol‐exposed mice. Hippocampus. 2021;31:3–10.
Mozzachiodi R, Byrne JH. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 2010;33:17–26.
Parsons RG. Behavioral and neural mechanisms by which prior experience impacts subsequent learning. Neurobiol Learn Memory. 2018;154:22–9.
Gouty-Colomer L, Hosseini B, Marcelo I, Schreiber J, Slump DE, Yamaguchi S, et al. Arc expression identifies the lateral amygdala fear memory trace. Mol Psych. 2016;21:364–75.
Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–40.
Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–5.
Lisman J, Cooper K, Sehgal M, Silva AJ. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci. 2018;21:309–14.
Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–7.
Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–9.
Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, et al. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–9.
Yu XW, Curlik DM, Oh MM, Yin JC, Disterhoft JF. CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. Elife. 2017;6:e19358.
Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–43.
Park S, Kramer EE, Mercaldo V, Rashid AJ, Insel N, Frankland PW, et al. Neuronal allocation to a hippocampal engram. Neuropsychopharmacology. 2016;41:2987–93.
Czajkowski R, Jayaprakash B, Wiltgen B, Rogerson T, Guzman-Karlsson MC, Barth AL, et al. Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci USA. 2014;111:8661–6.
Hsiang H-LL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N, et al. Manipulating a “cocaine engram” in mice. J Neurosci. 2014;34:14115–27.
Sano Y, Shobe JL, Zhou M, Huang S, Shuman T, Cai DJ, et al. CREB regulates memory allocation in the insular cortex. Curr Biol. 2014;24:2833–7.
Han J-H, Kushner SA, Yiu AP, Hsiang H-LL, Buch T, Waisman A, et al. Selective erasure of a fear memory. Science. 2009;323:1492–6.
Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–60.
Park A, Jacob AD, Walters BJ, Park S, Rashid AJ, Jung JH, et al. A time-dependent role for the transcription factor CREB in neuronal allocation to an engram underlying a fear memory revealed using a novel in vivo optogenetic tool to modulate CREB function. Neuropsychopharmacology. 2020;45:916–24.
Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang H-LL, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–35.
Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14.
Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–8.
O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5.
Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, et al. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci. 2015;18:1133–42.
Diamantaki M, Coletta S, Nasr K, Zeraati R, Laturnus S, Berens P, et al. Manipulating hippocampal place cell activity by single-cell stimulation in freely moving mice. Cell Rep. 2018;23:32–8.
Robinson NT, Descamps LA, Russell LE, Buchholz MO, Bicknell BA, Antonov GK, et al. Targeted activation of hippocampal place cells drives memory-guided spatial behavior. Cell. 2020;183:1586–99. e10.
Rich PD, Liaw H-P, Lee AK. Large environments reveal the statistical structure governing hippocampal representations. Science. 2014;345:814–7.
Epsztein J, Brecht M, Lee AK. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron. 2011;70:109–20.
Lee D, Lin B-J, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012;337:849–53.
Lisman J. Criteria for identifying the molecular basis of the engram (CaMKII, PKMzeta). Mol Brain. 2017;10:55.
Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15.
Frankland PW, Josselyn SA. Neuroscience: In search of the memory molecule. Nature. 2016;535:41–2.
Abraham WC, Jones OD, Glanzman DL. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci Learn. 2019;4:1–10.
Carillo-Reid L, Yuste R. What Is a Neuronal Ensemble? Oxford Research Encyclopedia of Neuroscience. 2020.
Carrillo-Reid L, Han S, Yang W, Akrouh A, Yuste R. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell. 2019;178:447–57. e5.
Carrillo-Reid L, Yang W, Bando Y, Peterka DS, Yuste R. Imprinting and recalling cortical ensembles. Science. 2016;353:691–4.
Alejandre-Garcia T, Kim S, Perez-Ortega J, Yuste R. Intrinsic excitability mechanisms of neuronal ensemble formation. Elife. 2022;11:e77470.
Chen L, Cummings KA, Mau W, Zaki Y, Dong Z, Rabinowitz S, et al. The role of intrinsic excitability in the evolution of memory: Significance in memory allocation, consolidation, and updating. Neurobiol Learn Memory. 2020;173:107266.
Sehgal M, Song C, Ehlers VL, Moyer JR Jr. Learning to learn–intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Memory. 2013;105:186–99.
Titley HK, Brunel N, Hansel C. Toward a neurocentric view of learning. Neuron. 2017;95:19–32.
Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900.
Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–45.
Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8.
Asok A, Leroy F, Rayman JB, Kandel ER. Molecular mechanisms of the memory trace. Trends Neurosci. 2019;42:14–22.
Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86.
Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annual Rev Neurosci. 1998;21:127–48.
Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde amnesia. Science. 2015;348:1007–13.
Rao-Ruiz P, Couey JJ, Marcelo IM, Bouwkamp CG, Slump DE, Matos MR, et al. Engram-specific transcriptome profiling of contextual memory consolidation. Nat Commun. 2019;10:1–14.
Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–7.
Choi J-H, Sim S-E, Kim J-I, Choi DI, Oh J, Ye S, et al. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018;360:430–5.
Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–3.
Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–2.
Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–94.
Buch ER, Claudino L, Quentin R, Bönstrup M, Cohen LG. Consolidation of human skill linked to waking hippocampo-neocortical replay. Cell Rep. 2021;35:109193.
Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–53.
Joo HR, Frank LM. The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nat Rev Neurosci. 2018;19:744–57.
Klinzing JG, Niethard N, Born J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. 2019;22:1598–610.
Dupret D, O’neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002.
Fernández-Ruiz A, Oliva A, de Oliveira EF, Rocha-Almeida F, Tingley D, Buzsáki G. Long-duration hippocampal sharp wave ripples improve memory. Science. 2019;364:1082–6.
Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–3.
Michon F, Sun J-J, Kim CY, Ciliberti D, Kloosterman F. Post-learning hippocampal replay selectively reinforces spatial memory for highly rewarded locations. Curr Biol. 2019;29:1436–44. e5.
Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–3.
Gridchyn I, Schoenenberger P, O’Neill J, Csicsvari J. Assembly-specific disruption of hippocampal replay leads to selective memory deficit. Neuron. 2020;106:291–300. e6.
Gillespie AK, Maya DAA, Denovellis EL, Liu DF, Kastner DB, Coulter ME, et al. Hippocampal replay reflects specific past experiences rather than a plan for subsequent choice. bioRxiv. 2021.
Schapiro AC, McDevitt EA, Rogers TT, Mednick SC, Norman KA. Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nat Commun. 2018;9:1–11.
Kim J, Kwon J-T, Kim H-S, Josselyn SA, Han J-H. Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci. 2014;17:65–72.
de Sousa AF, Cowansage KK, Zutshi I, Cardozo LM, Yoo EJ, Leutgeb S, et al. Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proc Natl Acad Sci USA. 2019;116:8576–81.
Ghandour K, Ohkawa N, Fung CCA, Asai H, Saitoh Y, Takekawa T, et al. Orchestrated ensemble activities constitute a hippocampal memory engram. Nat Commun. 2019;10:1–14.
Clawson BC, Pickup EJ, Ensing A, Geneseo L, Shaver J, Gonzalez-Amoretti J, et al. Causal role for sleep-dependent reactivation of learning-activated sensory ensembles for fear memory consolidation. Nat Commun. 2021;12:1–13.
Kumar D, Koyanagi I, Carrier-Ruiz A, Vergara P, Srinivasan S, Sugaya Y, et al. Sparse activity of hippocampal adult-born neurons during REM sleep is necessary for memory consolidation. Neuron. 2020;107:552–65. e10.
Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, et al. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–89.
Roy DS, Park YG, Kim ME, Zhang Y, Ogawa SK, DiNapoli N, et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat Commun. 2022;13:1799.
Gilboa A, Moscovitch M. No consolidation without representation: Correspondence between neural and psychological representations in recent and remote memory. Neuron. 2021;109:2239–55.
Wiltgen BJ, Tanaka KZ. Systems consolidation and the content of memory. Neurobiol Learn Memory. 2013;106:365–71.
Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–27.
Sekeres MJ, Winocur G, Moscovitch M. The hippocampus and related neocortical structures in memory transformation. Neurosci Lett. 2018;680:39–53.
Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal–neocortical interactions. Neuropsychologia. 2010;48:2339–56.
Richards BA, Xia F, Santoro A, Husse J, Woodin MA, Josselyn SA, et al. Patterns across multiple memories are identified over time. Nat Neurosci. 2014;17:981–6.
Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14:313–7.
Richards BA, Frankland PW. The persistence and transience of memory. Neuron. 2017;94:1071–84.
Yadav N, Noble C, Niemeyer JE, Terceros A, Victor J, Liston C, et al. Prefrontal feature representations drive memory recall. Nature. 2022;608:153–60.
Squire LR, Genzel L, Wixted JT, Morris RG. Memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021766.
Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, et al. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356:73–8.
Lee JH, Kim WB, Park EH, Cho JH. Neocortical synaptic engrams for remote contextual memories. Nat Neurosci. 2023;26:259–73.
Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9.
Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84:347–54.
Bota A, Goto A, Tsukamoto S, Schmidt A, Wolf F, Luchetti A, et al. Shared and unique properties of place cells in anterior cingulate cortex and hippocampus. bioRxiv. 2021.
Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29:8206–14.
Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–7.
Xia F, Richards BA, Tran MM, Josselyn SA, Takehara-Nishiuchi K, Frankland PW. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. Elife. 2017;6:e27868.
Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016;19:959–64.
Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80:352.
Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol. 2010;20:1336–44.
Richardson R, Riccio DC, Jonke T. Alleviation of infantile amnesia in rats by means of a pharmacological contextual state. Dev Psychobiol: J Int Soc Dev Psychobiol. 1983;16:511–8.
Goodwin DW, Powell B, Bremer D, Hoine H, Stern J. Alcohol and recall: State-dependent effects in man. Science. 1969;163:1358–60.
Godden DR, Baddeley AD. Context‐dependent memory in two natural environments: On land and underwater. Br J Psychol. 1975;66:325–31.
Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–4.
Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–3.
Gulmez Karaca K, Brito DVC, Kupke J, Zeuch B, Oliveira AMM. Engram reactivation during memory retrieval predicts long-term memory performance in aged mice. Neurobiol Aging. 2021;101:256–61.
Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–12.
Perusini JN, Cajigas SA, Cohensedgh O, Lim SC, Pavlova IP, Donaldson ZR, et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus. 2017;27:1110–22.
Rashid AJ, Yan C, Mercaldo V, Hsiang H-LL, Park S, Cole CJ, et al. Competition between engrams influences fear memory formation and recall. Science. 2016;353:383–7.
Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201.
Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22:1986–99.
DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Fu L, et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat Neurosci. 2019;22:460–9.
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–5.
Ramirez S, Liu X, Lin P-A, Suh J, Pignatelli M, Redondo RL, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–91.
Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, et al. Generation of a synthetic memory trace. Science. 2012;335:1513–6.
Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–30.
Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–41.
Pignatelli M, Ryan TJ, Roy DS, Lovett C, Smith LM, Muralidhar S, et al. Engram cell excitability state determines the efficacy of memory retrieval. Neuron. 2019;101:274–84 e5.
Guskjolen A, Kenney JW, de la Parra J, Yeung B-RA, Josselyn SA, Frankland PW. Recovery of “lost” infant memories in mice. Curr Biol. 2018;28:2283–90.
Tulving E. Cue-dependent forgetting: When we forget something we once knew, it does not necessarily mean that the memory trace has been lost; it may only be inaccessible. Am Sci. 1974;62:74–82.
Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–61.
Klein SB, Robertson TE, Delton AW. Facing the future: Memory as an evolved system for planning future acts. Mem Cogn. 2010;38:13–22.
Hardt O, Nader K, Nadel L. Decay happens: the role of active forgetting in memory. Trends Cogn Sci. 2013;17:111–20.
Davis RL, Zhong Y. The biology of forgetting—a perspective. Neuron. 2017;95:490–503.
Nørby S. Why forget? On the adaptive value of memory loss. Perspect Psychol Sci. 2015;10:551–78.
Kraemer PJ, Golding JM. Adaptive forgetting in animals. Psych Bull Rev. 1997;4:480–91.
Abraham WC, Robins A. Memory retention–the synaptic stability versus plasticity dilemma. Trends Neurosci. 2005;28:73–8.
Sweis BM, Mau W, Rabinowitz S, Cai DJ. Dynamic and heterogeneous neural ensembles contribute to a memory engram. Curr Opin Neurobiol. 2021;67:199–206.
Ziv NE, Brenner N. Synaptic tenacity or lack thereof: spontaneous remodeling of synapses. Trends Neurosci. 2018;41:89–99.
Ryan TJ, Frankland PW. Forgetting as a form of adaptive engram cell plasticity. Nat Rev Neurosci. 2022;23:173–86.
Adlaf EW, Vaden RJ, Niver AJ, Manuel AF, Onyilo VC, Araujo MT, et al. Adult-born neurons modify excitatory synaptic transmission to existing neurons. Elife. 2017;6:e19886.
Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–7.
Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–34.
Guskjolen A. Engrams, Neurogenesis, and Forgetting University of Toronto; 2019.
Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang H-LL, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602.
Epp JR, Mera RS, Köhler S, Josselyn SA, Frankland PW. Neurogenesis-mediated forgetting minimizes proactive interference. Nat Commun. 2016;7:1–8.
Gao A, Xia F, Guskjolen AJ, Ramsaran AI, Santoro A, Josselyn SA, et al. Elevation of hippocampal neurogenesis induces a temporally graded pattern of forgetting of contextual fear memories. J Neurosci. 2018;38:3190–8.
Ishikawa R, Fukushima H, Frankland PW, Kida S. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. Elife. 2016;5:e17464.
Scott GA, Terstege DJ, Roebuck AJ, Gorzo KA, Vu AP, Howland JG, et al. Adult neurogenesis mediates forgetting of multiple types of memory in the rat. Molecular Brain. 2021;14:97–109.
Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020;367:688–94.
Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182:388–403. e15.
Wang Z, Chen R, Lin Q, Jiang Y, Le Q, Liu X, et al. Autophagy in DG engrams mediates Rac1-dependent forgetting via TLR2/4 signals in microglia. bioRxiv. 2022.
Lee J-H, Kim J-Y, Noh S, Lee H, Lee SY, Mun JY, et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature. 2021;590:612–7.
Navarrete M, Cuartero MI, Palenzuela R, Draffin JE, Konomi A, Serra I, et al. Astrocytic p38α MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat Commun. 2019;10:1–15.
Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–69.
Migues PV, Wong J, Lyu J, Hardt O. NMDA receptor activity bidirectionally controls active decay of long‐term spatial memory in the dorsal hippocampus. Hippocampus. 2019;29:883–8.
Sachser RM, Santana F, Crestani AP, Lunardi P, Pedraza LK, Quillfeldt JA, et al. Forgetting of long-term memory requires activation of NMDA receptors, L-type voltage-dependent Ca 2+ channels, and calcineurin. Sci Rep. 2016;6:1–9.
Villarreal DM, Do V, Haddad E, Derrick BE. NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat Neurosci. 2002;5:48–52.
Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, et al. PKMζ maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–4.
Dong Z, Han H, Li H, Bai Y, Wang W, Tu M, et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J Clin Investig. 2015;125:234–47.
Migues PV, Liu L, Archbold GE, Einarsson EÖ, Wong J, Bonasia K, et al. Blocking synaptic removal of GluA2-containing AMPA receptors prevents the natural forgetting of long-term memories. J Neurosci. 2016;36:3481–94.
Guskjolen AJ. Losing connections, losing memory: Ampa receptor endocytosis as a neurobiological mechanism of forgetting. J Neurosci. 2016;36:7559–61.
Awasthi A, Ramachandran B, Ahmed S, Benito E, Shinoda Y, Nitzan N, et al. Synaptotagmin-3 drives AMPA receptor endocytosis, depression of synapse strength, and forgetting. Science. 2019;363:eaav1483.
Wu W, Du S, Shi W, Liu Y, Hu Y, Xie Z, et al. Inhibition of Rac1-dependent forgetting alleviates memory deficits in animal models of Alzheimer’s disease. Prot Cell. 2019;10:745–59.
Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y. Forgetting is regulated through Rac activity in Drosophila. Cell. 2010;140:579–89.
Cervantes-Sandoval I, Chakraborty M, MacMullen C, Davis RL. Scribble scaffolds a signalosome for active forgetting. Neuron. 2016;90:1230–42.
Liu Y, Du S, Lv L, Lei B, Shi W, Tang Y, et al. Hippocampal activation of Rac1 regulates the forgetting of object recognition memory. Curr Biol. 2016;26:2351–7.
Liu Y, Lv L, Wang L, Zhong Y. Social iIsolation induces Rac1-dependent forgetting of social memory. Cell Rep. 2018;25:288–95 e3.
Kikuchi M, Sekiya M, Hara N, Miyashita A, Kuwano R, Ikeuchi T, et al. Disruption of a RAC1-centred network is associated with Alzheimer’s disease pathology and causes age-dependent neurodegeneration. Human Mol Gen. 2020;29:817–33.
Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–40.
de Oliveira Alvares L, Do-Monte FH. Understanding the dynamic and destiny of memories. Neurosci Biobehav Rev. 2021;125:592–607.
Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–8.
Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu S-i, et al. Synapse-specific representation of the identity of overlapping memory engrams. Science. 2018;360:1227–31.
Kim WB, Cho J-H. Encoding of discriminative fear memory by input-specific LTP in the amygdala. Neuron. 2017;95:1129–46. e5.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–52.
Brun VH, Ytterbø K, Morris RG, Moser M-B, Moser EI. Retrograde amnesia for spatial memory induced by NMDA receptor-mediated long-term potentiation. J Neurosci. 2001;21:356–62.
Poll S, Mittag M, Musacchio F, Justus LC, Giovannetti EA, Steffen J, et al. Memory trace interference impairs recall in a mouse model of Alzheimer’s disease. Nat Neurosci. 2020;23:952–8.
Iwasaki S, Ikegaya Y. Contextual fear memory retrieval is vulnerable to hippocampal noise. Cerebral Cortex. 2021;31:785–94.
Krueger JN, Wilmot JH, Teratani-Ota Y, Puhger KR, Nemes SE, Crestani AP, et al. Amnesia for context fear is caused by widespread disruption of hippocampal activity. Neurobiol Learn Memory. 2020;175:107295.
Tulving E, Pearlstone Z. Availability versus accessibility of information in memory for words. J Verbal Learn Verbal Behav. 1966;5:381–91.
Bessières B, Travaglia A, Mowery TM, Zhang X, Alberini CM. Early life experiences selectively mature learning and memory abilities. Nat Commun. 2020;11:1–16.
Li S, Richardson R. Traces of memory: reacquisition of fear following forgetting is NMDAr-independent. Learn Memory. 2013;20:174–82.
Kim JH, McNally GP, Richardson R. Recovery of fear memories in rats: role of gamma-amino butyric acid (GABA) in infantile amnesia. Behav Neurosci. 2006;120:40.
Travaglia A, Bisaz R, Sweet ES, Blitzer RD, Alberini CM. Infantile amnesia reflects a developmental critical period for hippocampal learning. Nat Neurosci. 2016;19:1225–33.
Chen S, Cai D, Pearce K, Sun PY, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife. 2014;3:e03896.
Ortega de San Luis C, Ryan TJ. Understanding the physical basis of memory: Molecular Mechanisms of the Engram. J Biol Chem. 2022;298:101866.
Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–8.
Gold AR, Glanzman DL. The central importance of nuclear mechanisms in the storage of memory. Biochem Biophys Res Commun. 2021;564:103–13.
Langille JJ, Gallistel CR. Locating the engram: Should we look for plastic synapses or information-storing molecules? Neurobiol Learn Mem. 2020;169:107164.
Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–66.
Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell. 2018;174:59–71. e14.
Doron A, Rubin A, Benmelech-Chovav A, Benaim N, Carmi T, Refaeli R, et al. Hippocampal astrocytes encode reward location. Nature. 2022;609:772–8.
Vetere G, Xia F, Ramsaran AI, Tran LM, Josselyn SA, Frankland PW. An inhibitory hippocampal–thalamic pathway modulates remote memory retrieval. Nat Neurosci. 2021;24:685–93.
Nomura H, Hara K, Abe R, Hitora-Imamura N, Nakayama R, Sasaki T, et al. Memory formation and retrieval of neuronal silencing in the auditory cortex. Proc Natl Acad Sci USA. 2015;112:9740–4.
Cummings KA, Bayshtok S, Dong TN, Kenny PJ, Clem RL. Control of fear by discrete prefrontal GABAergic populations encoding valence-specific information. Neuron. 2022;110:3036–52.e5.
Cummings KA, Clem RL. Prefrontal somatostatin interneurons encode fear memory. Nat Neurosci. 2020;23:61–74.
Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, et al. Distinct neural circuits for the formation and retrieval of episodic memories. Cell. 2017;170:1000–12. e19.
Quiñones-Laracuente K, Vega-Medina A, Quirk GJ. Time-dependent recruitment of prelimbic prefrontal circuits for retrieval of fear memory. Front Behav Neurosci. 2021;15:665116.
Cembrowski MS, Phillips MG, DiLisio SF, Shields BC, Winnubst J, Chandrashekar J, et al. Dissociable structural and functional hippocampal outputs via distinct subiculum cell classes. Cell. 2018;173:1280–92. e18.
Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72.
Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–34.
Favila SE, Lee H, Kuhl BA. Transforming the concept of memory reactivation. Trends Neurosci. 2020;43:939–50.
Hardt O, Sossin WS. Terminological and epistemological issues in current memory research. Front Mol Neurosci. 2019;12:336.
Roediger HL 3rd. Memory metaphors in cognitive psychology. Mem Cognit. 1980;8:231–46.
Vilarroya O. Neural representation. A survey-based analysis of the notion. Front Psychol. 2017;8:1458.
Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387.
Sehgal M, Song C, Ehlers VL, Moyer JR Jr. Learning to learn - intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem. 2013;105:186–99.
Yousuf H, Ehlers VL, Sehgal M, Song C, Moyer JR Jr. Modulation of intrinsic excitability as a function of learning within the fear conditioning circuit. Neurobiol Learn Mem. 2020;167:107132.
Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, et al. Synaptic tagging during memory allocation. Nat Rev Neurosci. 2014;15:157–69.
Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703.
Lavi A, Sehgal M, de Sousa AF, Ter-Mkrtchyan D, Sisan F, Luchetti A, et al. Local memory allocation recruits memory ensembles across brain regions. Neuron. 2023;111:470–80.
Palacios-Filardo J, Mellor JR. Neuromodulation of hippocampal long-term synaptic plasticity. Curr Opin Neurobiol. 2019;54:37–43.
Brzosko Z, Mierau SB, Paulsen O. Neuromodulation of spike-timing-dependent plasticity: past, present, and future. Neuron. 2019;103:563–81.
Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behav Neurosci. 1986;100:147–54.
Teyler TJ, Rudy JW. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus. 2007;17:1158–69.
Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–41.
Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–68.
Tanaka KZ, McHugh TJ. The hippocampal engram as a memory index. J Exp Neurosci. 2018;12:1179069518815942.
Goode TD, Tanaka KZ, Sahay A, McHugh TJ. An integrated index: engrams, place cells, and hippocampal memory. Neuron. 2020;107:805–20.
Sun X, Bernstein MJ, Meng M, Rao S, Sorensen AT, Yao L, et al. Functionally distinct neuronal ensembles within the memory engram. Cell. 2020;181:410–23 e17.
Moore RS, Kaletsky R, Lesnik C, Cota V, Blackman E, Parsons LR, et al. The role of the Cer1 transposon in horizontal transfer of transgenerational memory. Cell. 2021;184:4697–712.e18.
Bedecarrats A, Chen S, Pearce K, Cai D, Glanzman DL. RNA from trained aplysia can induce an epigenetic engram for long-term sensitization in untrained aplysia. eNeuro. 2018;5:ENEURO.0038–18.2018.
Kaletsky R, Moore RS, Vrla GD, Parsons LR, Gitai Z, Murphy CT. C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance. Nature. 2020;586:445–51.
Moore RS, Kaletsky R, Murphy CT. Piwi/PRG-1 argonaute and TGF-beta mediate transgenerational learned pathogenic avoidance. Cell. 2019;177:1827–41.e12.
Posner R, Toker IA, Antonova O, Star E, Anava S, Azmon E, et al. Neuronal small RNAs control behavior transgenerationally. Cell. 2019;177:1814–26.e15.
Bozler J, Kacsoh BZ, Bosco G. Transgeneratonal inheritance of ethanol preference is caused by maternal NPF repression. Elife. 2019;8:e45391.
Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96.
Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16:42–7.
Campbell RR, Wood MA. How the epigenome integrates information and reshapes the synapse. Nat Rev Neurosci. 2019;20:133–47.
Zovkic IB. Epigenetics and memory: an expanded role for chromatin dynamics. Curr Opin Neurobiol. 2021;67:58–65.
Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci USA. 2013;110:12456–61.
Blackiston DJ, Shomrat T, Levin M. The stability of memories during brain remodeling: A perspective. Commun Integr Biol. 2015;8:e1073424.
Acknowledgements
M.S.C. is supported by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research. AG is supported by a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship.
Author information
Authors and Affiliations
Contributions
AG and MSC conceptualized, wrote, and revised the manuscript and figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guskjolen, A., Cembrowski, M.S. Engram neurons: Encoding, consolidation, retrieval, and forgetting of memory. Mol Psychiatry 28, 3207–3219 (2023). https://doi.org/10.1038/s41380-023-02137-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02137-5
This article is cited by
-
Overnight neuronal plasticity and adaptation to emotional distress
Nature Reviews Neuroscience (2024)
-
Intermediate-term memory mechanism inspired lightweight single image super resolution
Multimedia Tools and Applications (2024)