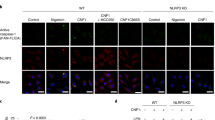
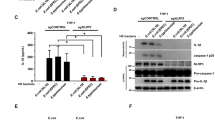
Abstract
Caspase-11 detection of intracellular lipopolysaccharide (LPS) from invasive Gram-negative bacteria mediates noncanonical activation of the NLRP3 inflammasome. While avirulent bacteria do not invade the cytosol, their presence in tissues necessitates clearance and immune system mobilization. Despite sharing LPS, only live avirulent Gram-negative bacteria activate the NLRP3 inflammasome. Here, we found that bacterial mRNA, which signals bacterial viability, was required alongside LPS for noncanonical activation of the NLRP3 inflammasome in macrophages. Concurrent detection of bacterial RNA by NLRP3 and binding of LPS by pro-caspase-11 mediated a pro-caspase-11–NLRP3 interaction before caspase-11 activation and inflammasome assembly. LPS binding to pro-caspase-11 augmented bacterial mRNA-dependent assembly of the NLRP3 inflammasome, while bacterial viability and an assembled NLRP3 inflammasome were necessary for activation of LPS-bound pro-caspase-11. Thus, the pro-caspase-11–NLRP3 interaction nucleated a scaffold for their interdependent activation explaining their functional reciprocal exclusivity. Our findings inform new vaccine adjuvant combinations and sepsis therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Datasets generated or analyzed during this study are available on reasonable request. Source data are provided with this paper.
References
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Lieberman, J., Wu, H. & Kagan, J. C. Gasdermin D activity in inflammation and host defense. Sci. Immunol. 4, eaav1447 (2019).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Case, C. L. et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl Acad. Sci. USA 110, 1851–1856 (2013).
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Blander, J. M. & Sander, L. E. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat. Rev. Immunol. 12, 215–225 (2012).
Finethy, R. et al. Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. mBio 8, e01188–17 (2017).
Sander, L. E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011).
Barbet, G. et al. Sensing microbial viability through bacterial RNA augments t follicular helper cell and antibody responses. Immunity 48, 584–598 e585 (2018).
Moretti, J. et al. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell 171, 809–823 e813 (2017).
Blander, J. M. & Barbet, G. Exploiting vita-PAMPs in vaccines. Curr. Opin. Pharmacol. 41, 128–136 (2018).
Hayward, J. A., Mathur, A., Ngo, C. & Man, S. M. Cytosolic recognition of microbes and pathogens: inflammasomes in action. Microbiol. Mol. Biol. Rev. 82, e00015–e00018 (2018).
Kailasan Vanaja, S. et al. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 111, 7765–7770 (2014).
Gros, M. & Amigorena, S. Regulation of antigen export to the cytosol during cross-presentation. Front Immunol. 10, 41 (2019).
Herskovits, A. A., Auerbuch, V. & Portnoy, D. A. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 3, e51 (2007).
Nakamura, N. et al. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509, 240–244 (2014).
Dick, M. S., Sborgi, L., Ruhl, S., Hiller, S. & Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 7, 11929 (2016).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006).
Stutz, A., Horvath, G. L., Monks, B. G. & Latz, E. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol. 1040, 91–101 (2013).
Sanchez-Garrido, J., Slater, S. L., Clements, A., Shenoy, A. R. & Frankel, G. Vying for the control of inflammasomes: the cytosolic frontier of enteric bacterial pathogen-host interactions. Cell Microbiol. 22, e13184 (2020).
Lu, A. et al. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat. Struct. Mol. Biol. 23, 416–425 (2016).
Chen, J. & Chen, Z. J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564, 71–76 (2018).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Sasaki, H. & White, S. H. Aggregation behavior of an ultra-pure lipopolysaccharide that stimulates TLR-4 receptors. Biophys. J. 95, 986–993 (2008).
Sanders, M. G. et al. Single-cell imaging of inflammatory caspase dimerization reveals differential recruitment to inflammasomes. Cell Death Dis. 6, e1813 (2015).
Santos, J. C. et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 (2020).
Wandel, M. P. et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 21, 880–891 (2020).
Kutsch, M. et al. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 39, e104926 (2020).
An, J. et al. Caspase-4 disaggregates lipopolysaccharide micelles via LPS-CARD interaction. Sci. Rep. 9, 826 (2019).
Aurell, C. A. & Wistrom, A. O. Critical aggregation concentrations of gram-negative bacterial lipopolysaccharides (LPS). Biochem. Biophys. Res. Commun. 253, 119–123 (1998).
Bergstrand, A., Svanberg, C., Langton, M. & Nyden, M. Aggregation behavior and size of lipopolysaccharide from Escherichia coli O55:B5. Colloids Surf. B Biointerfaces 53, 9–14 (2006).
Santos, N. C., Silva, A. C., Castanho, M. A., Martins-Silva, J. & Saldanha, C. Evaluation of lipopolysaccharide aggregation by light scattering spectroscopy. ChemBioChem 4, 96–100 (2003).
Belasco, J. G. All things must pass: contrasts and commonalities in eukaryotic and bacterial mRNA decay. Nat. Rev. Mol. Cell Biol. 11, 467–478 (2010).
Eigenbrod, T. et al. Bacterial RNA mediates activation of caspase-1 and IL-1beta release independently of TLRs 3, 7, 9 and TRIF but is dependent on UNC93B. J. Immunol. 189, 328–336 (2012).
Sha, W. et al. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc. Natl Acad. Sci. USA 111, 16059–16064 (2014).
Ugolini, M. et al. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat. Immunol. 19, 386–396 (2018).
Deets, K. A. & Vance, R. E. Inflammasomes and adaptive immune responses. Nat. Immunol. 22, 412–422 (2021).
Munoz-Wolf, N. & Lavelle, E. C. A Guide to IL-1 family cytokines in adjuvanticity. FEBS J. 285, 2377–2401 (2018).
Cavaillon, J. M., Singer, M. & Skirecki, T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 12, e10128 (2020).
Reynolds, C. M. & Raetz, C. R. Replacement of lipopolysaccharide with free lipid A molecules in Escherichia coli mutants lacking all core sugars. Biochemistry 48, 9627–9640 (2009).
Buchrieser, C. et al. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunol. Med. Microbiol. 35, 207–213 (2003).
Cheung, A. L., Bayer, A. S., Zhang, G., Gresham, H. & Xiong, Y. Q. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40, 1–9 (2004).
Moretti, J., Vabret, N. & Blander, J. M. Measuring innate immune responses to bacterial viability. Methods Mol. Biol. 1714, 167–190 (2018).
Nierlich, D. P. Regulation of ribonucleic acid synthesis in growing bacterial cells. II. Control over the composition of the newly made RNA. J. Mol. Biol. 72, 765–777 (1972).
Lugrin, J. & Martinon, F. Detection of ASC oligomerization by western blotting. Bio Protoc. 7, e2292 (2017).
Ramsby, M. & Makowski, G. Differential detergent fractionation of eukaryotic cells. Cold Spring Harb. Protoc. 2011, prot5592 (2011).
Strack, R. A peck of peppers. Nat. Methods 16, 1075 (2019).
Wu, J. et al. Live imaging of mRNA using RNA-stabilized fluorogenic proteins. Nat. Methods 16, 862–865 (2019).
Bryan, N. B., Dorfleutner, A., Rojanasakul, Y. & Stehlik, C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182, 3173–3182 (2009).
Acknowledgements
We are grateful to D. Amsen, N. Vabret, T. Kanneganti, K. Fitzgerald, V. Dixit, F. Shao, M. Goldberg, R. Ernst, J. Chipuk, J. Gelles-Hurwitz and R. Flavell for reagents and discussions. We thank D. Filipescu, C. Brou, P. Chastagner, A. Israël, A. Zanin-Zhorov, S. Waksal, M.A. Blander and S.J. Blander for their support. This work was supported by National Institutes of Health (NIH) grants AI127658 to J.M.B., AI139425 to J.C., R35NS111631 to S.R.J. and Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease Awards to J.M.B and to J.C. The Blander laboratory was supported by NIH grants DK072201, DK111862, AI095245 and AI123284 to J.M.B. J.M.B. was supported by the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Contributions
J.M. and J.M.B. directed the study, designed experiments and wrote the manuscript. J.M. performed most experiments, data and statistical analyses and all macrophage stimulations. B.J. performed molecular biology, cloning, transfection and ViewRNA ISH related experiments. Z.H. conducted the experiments related to measuring RNAbac in cytosolic extracts and kinetics of macrophage cell death. S.R. performed experiments related to Fig. 1c,f and the dual LPS and RNAbac requirement for noncanonical NLRP3 inflammasome activation during early stages of the work. M.S. conducted confocal microscopy lysosomal localization of bacteria and quantification. H.Y. performed experiments related to Extended Data Fig. 8c. J.W. and S.R.J. provided expertise on Pepper RNA-regulated fluorogenic protein stabilization. J.C. provided conceptual advice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Ioana Visan was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bacterial mRNA and stimulatory LPS are both required for caspase-11 and noncanonical inflammasome activation.
a, Immunofluorescence confocal microscopy on bone marrow derived macrophages (hereafter ‘macrophages’) 2 h post stimulation with L or HK E. coli or untreated. b, Bar graph, % cells with LAMP1-LPS colocalization. c, Immunoblots of macrophage concentrated supernatants (20 h) or WCE (6 h), and cytokine concentrations and LDH release in culture supernatants (20 h) post stimulation with L, HK or HK E. coli supplemented with 100 ng ml–1 mRNA isolated from E. coli or eukaryotic cells. LDH measured by cytotoxicity assay; IL-1β, TNF and IL-6 by ELISA. Error bars, mean ± s.e.m. t-test was performed in b (n = 3). ns: nonsignificant. Bacteria:macrophage=20:1. Results represent at least three independent experiments.
Extended Data Fig. 2 Cytosolic levels of LPS and bacterial mRNA after macrophage stimulation with live or heat-killed bacteria.
a, Measurements of LPS in the cytosolic (C) or residual (R) fractions prepared from macrophages 2 hr post-transfection of indicated doses of LPS alone, co-transfection of 2 ng ml–1 LPS with 100 ng ml–1 E. coliLPSmut mRNA, or stimulation with L or HK E. coli or virulent E. coli as indicated. LPS was measured via Limulus Amebocyte Lysate method. b, Immunoblots of cytosolic (C) and residual (R) fractions from macrophages 2 h post stimulation with L E. coli, HK E. coli or L virulent E. coli, and probed with marker antibodies for indicated subcellular compartments. c, RT–qPCR for the bacterial gene groES, groEL, era and dnaE on cytosolic fractions prepared 2 h post macrophage transfection with indicated doses of ultrapure LPS and mRNA prepared from E. coli, or stimulation with L, HK or HK E. coli or virulent E. coli supplemented with mRNA prepared from each bacterium. d,e, mNeonGreen fluorescence measurements on total extracts (d) or cytosolic versus residual fractions (e) of macrophages stably expressing tDeg-tagged mNeonGreen 2 h post stimulation with E. coli expressing or not Pepper RNA, or after treatment with proteasome inhibitor MG132 as indicated. By expressing a Pepper RNA-regulated fluorogenic protein (mNeonGreen-tDeg) in the cytosol of macrophages, we noted 1.5-2-fold increase in mNeonGreen fluorescence following phagocytosis of recombinant Pepper RNA-expressing E. coli compared to the 2-2.5-fold increase with the proteasomal inhibitor MG132, indicating cytosolic access of Pepper RNA derived from phagocytosed E. coli bound to and stabilized the fluorogenic protein in the cytosol of macrophages. f,h, Confocal microscopy of direct fluorescence RNA in situ hybridization (ViewRNA ISH) to detect two RNAbac transcripts encoding for either mCherry (f) or endogenous GroES (h) from recombinant mCherry E. coli showed significantly more probe signal in macrophages at 6 h post-phagocytosis of live (L) compared with killed (K) bacteria. Killed bacteria were visualized by anti-LPS staining due to loss of mCherry fluorescence. RNA probe signal localized with live bacteria in lysosomes labeled with LAMP-1 as expected, but almost half of this signal did not colocalize to these bacteria suggesting cytosolic access. Inserts show magnification of indicated area. g,i, Bar graphs show quantification of probe signal in (f) and (h), respectively. Bacteria:macrophage=20:1. Error bars, mean ± s.e.m. One-way ANOVA followed by multiple comparisons Sidak tests and p values are indicated in bar graphs in g (Total number, Untreated: n = 13, L: n = 19, K: n = 15, K(LPS): n = 17 – Not colocalized, Untreated: n = 11, L, K: n = 14) and i (Total number, Untreated: n = 11, L: n = 14, K, K(LPS): n = 10 – Not colocalized, n = 10). Results represent at least three independent experiments.
Extended Data Fig. 3 Bacterial mRNA and LPS are both required for IL-1β secretion and generation of the active form of caspase-11.
a, Cytokine concentrations and LDH release in culture supernatants (20 h) post-transfection of WT or Nlrp3–/– macrophages as indicated with 2 ng ml–1 ultrapure LPS and/or 10, 30 and 100 ng ml–1 of mRNA prepared from E. coli LPSmut, L. innocua, or in vitro transcribed (IVT). b, Immunoblots of macrophage concentrated supernatants, WCE, mixed concentrated supernatants and WCE, or pulldown of caspases with biotinylated zVAD-FMK from WT, Nlrp3–/– or Casp11–/– macrophages 20 h post stimulation with L, HK or HK E. coli supplemented with E. coli total RNA (RNAtot), or transfection with indicated doses of ultrapure LPS and E. coli RNAtot. c, In vitro zVAD-AMC fluorescence post-incubation with immunoprecipitates of endogenous caspase-11 from macrophages stimulated for 6 h or 12 h as indicated with L, HK, or HK E. coli supplemented with E. coli RNAtot (10 µg ml–1). No IgG served as a control for Protein G-bound proteins alone. Bacteria:macrophage=20:1. Error bars, mean ± s.e.m. Results represent at least three independent experiments.
Extended Data Fig. 4 LPS is required alongside bacterial mRNA for assembly of the NLRP3 inflammasome and regardless of LPS-stimulatory activity.
a–c, Immunofluorescence confocal microscopy on macrophages 16 h post stimulation of indicated macrophage genotypes with L, gentamicin-killed (K) or K red fluorescent protein (RFP) expressing recombinant E. coli supplemented with E. coli RNAtot. Note, the partial nuclear staining pattern has previously been observed (see Methods) and appears to be a specific signal given its absence in Pycard–/– macrophages. In a,b, insets show magnification of indicated areas. White arrowheads point to ASC specks. Phalloidin delineates the macrophage actin cytoskeleton. Scale bar=10 µm. Bar graphs, % cells exhibiting ASC specks. Error bars, mean ± s.e.m. t-test was performed in b (n = 5). One-way ANOVA followed by multiple comparisons Sidak tests were performed in a (n = 5) and c (n = 6). P values are indicated in bar graphs. Bacteria:macrophage=20:1. Results represent at least three independent experiments.
Extended Data Fig. 5 Kinetics of noncanonical activation of the NLPR3 inflammasome in macrophages in response to virulent and avirulent bacteria.
a, LDH released in culture supernatants of macrophages at indicated time points post-stimulation with Live E. coli (avirulent), virulent E. coli or virulent Salmonella. LDH measured by cytotoxicity assay. b,c, Kinetics of SYTOX Red incorporation over 72 h in macrophages stimulated with Live E. coli or virulent E. coli, Salmonella ΔSpi1/2 (avirulent) or wild type (WT) (virulent), or Shigella BS103 (avirulent) or WT (virulent) (b), and representative images of SYTOX Red incorporation at selected time points (c). Peaks of SYTOX incorporation occurred faster (blue boxes) in response to virulent bacteria, before decreasing likely due to destruction of cell structure, while macrophages stimulated with avirulent bacteria were still incorporating SYTOX and peaked later (orange boxes). Scale bar=150 µm. d, Counting of live macrophages at the indicated time points post stimulation with Live E. coli or virulent E. coli. Counts were normalized to the unstimulated macrophages for each time point. e, Immunoblots of macrophage WCE at the indicated time points post stimulation with Live E. coli, virulent E. coli or virulent Salmonella. f, Immunoblots of macrophage concentrated supernatants 6 h post stimulation with Live E. coli or virulent E. coli. Error bars, mean ± s.e.m. Bacteria:macrophage =20:1 for E. coli and virulent E. coli, 5:1 for Salmonella and Shigella strains. Results represent at least three independent experiments.
Extended Data Fig. 6 NLRP3 stimuli specifically couple with LPS in mediating noncanonical activation of the NLRP3 inflammasome.
a,b, Immunoblots of macrophage concentrated supernatants (20 h), WCE (6 h), or cross-linked fractions (16 h), and cytokine concentrations in culture supernatants (20 h) as indicated post stimulation with L, HK or HK E. coli supplemented with indicated doses of RNAtot or Nigericin (a), and post-treatment with Nigericin or transfection of indicated doses of poly(dA:dT) or Flagellin with or without prior priming with 100 ng ml–1 LPS for 12–16 h (b). IL-1β and IL-6 by ELISA. Error bars, mean ± s.e.m.
Extended Data Fig. 7 dTGN colocalization and biochemical pro-caspase-11–NLRP3 interaction.
a,b, Immunofluorescence confocal microscopy 16 h post stimulation of WT macrophages with L or HK virulent E. coli (a), and WT, Casp11–/– and Nlrp3–/– macrophages as indicated with L E coli (b). Scale bar=10 µm. In (a), side micrograph insets in the triple merges show magnification of the indicated areas. c,d, Immunoprecipitation (IP) of endogenous caspase-11 or NLRP3 as indicated, and immunoblotting for co-immunoprecipitating proteins and WCE proteins (labels to left of immunoblot panels) from macrophages stimulated 12 h with L, HK or HK E. coli supplemented with indicated dose of E. coli RNAtot. (c) and L, HK or HK E. coli supplemented with indicated dose of E. coli RNAtot or Nigericin, E. coli RNAtot alone or Nigericin alone (d). Bacteria:macrophage=20:1. Results represent at least three independent experiments.
Extended Data Fig. 8 GSDMD is important for noncanonical activation of the NLRP3 inflammasome in response to Gram-negative bacteria.
a–c, Immunoblots of macrophage concentrated supernatants (20 h) or WCE at 6 h (a,b) and 20 h (c) post stimulation of WT and Gsdmd–/– macrophages with L or HK E. coli (avirulent) or virulent E. coli as indicated. (d) Immunoblots of macrophage concentrated supernatants (20 h) or WCE (6 h) post stimulation of macrophages with L or HK E. coli with or without zVAD-FMK treatment.
Extended Data Fig. 9 Model for noncanonical activation of the NLRP3 inflammasome by live Gram-negative bacteria.
Following phagocytosis of live Gram-negative bacteria, two classes of PAMPs are exposed to cytoplasmic pattern recognition receptors: the classical PAMP LPS, shared by live and killed bacteria, and the vita-PAMP bacterial mRNA (mRNAbac), present only in live bacteria. Coincident detection of mRNAbac and LPS from virulent or avirulent Gram-negative bacteria alike promotes a physical and mutually exclusive interaction between NLRP3 and the intracellular LPS receptor pro-caspase-11. This interaction localizes to the dispersed Trans-Golgi Network (TGN) and is mediated through the pro-caspase-11 SCAF domain and the LRR and PYD domains of NLRP3. The interaction of NLRP3 and pro-caspase-11 is upstream of their activation: It does not require the ability of LPS to activate caspase-11 and can still occur in the absence of GSDMD. It also does not require ASC and caspase-1 which are important for NLRP3 activation. Besides their interaction, NLRP3 and pro-caspase-11 are reciprocally required for their function: LPS binding to but not activation of pro-caspase-11, is necessary for mRNAbac-mediated NLRP3 inflammasome assembly. Reciprocally, NLRP3 and ASC but not caspase-1 are required for pro-caspase-11 activation, indicating the necessity for ‘nascent’ NLRP3 inflammasome assembly upon sensing the viability of Gram-negative bacteria (detection of mRNAbac) and irrespective of bacterial virulence factor expression. Although NLRP3-ASC oligomers can form in the absence of pro-caspase-1, these oligomers are unstable indicating stabilization of the ‘nascent’ assembled NLRP3 inflammasome upon pro-caspase-1 recruitment. Furthermore, higher concentrations of intracellular LPS, likely due to virulence factor activity during infection with virulent cell-invasive Gram-negative bacteria, trigger faster kinetics of plasma membrane permeabilization/pyroptosis compared with avirulent bacteria, and independently of NLRP3 and ASC2,9. Collectively, the model that emerges demonstrates two modes of pro-caspase-11 activation by LPS, a fast NLRP3-independent mode triggered by the concurrent expression of bacterial virulence factors, and a slower NLRP3-dependent mode triggered by coincident detection of the vita-PAMP mRNAbac that signifies bacterial viability. (SCAF, scaffold; PYD, Pyrin; LRR, leucine rich repeat).
Extended Data Fig. 10 Characterization and mapping of the pro-caspase-11 and NLRP3 interaction.
a, Schematic indicating the different truncated mutants of NLRP3 used for co-immunoprecipitation experiments in 293 T cells. All forms were fused to 3xHA tag in N-Terminus. b, Schematic indicating the different truncated mutants of casp11C254A used for co-immunoprecipitation experiments in 293 T cells. All forms were fused to 3xFLAG tag in N-Terminus. c, Immunoprecipitation and immunoblotting for coimmunoprecipitating and WCE proteins of overexpressed FLAG-caspase-11 either casp11C254A FL, Casp-11 C254A ΔCARD or Casp-11 C254A ΔSCAF with or without HA-NLRP3FL in 293 T cells 24 h post-transfection. To equilibrate the levels of FLAG-caspase-11 mutants in the immunoprecipitates, 4 times more protein extracts were submitted to anti-FLAG immunoprecipitation when FLAG-casp11C254A ΔSCAF was expressed (labelled 4X) compared to when either FLAG-casp11C254A FL or FLAG-casp11C254A ΔCARD were expressed (labelled 1X). Therefore, note that all proteins from FLAG-casp11C254A ΔSCAF samples (including HA-NLRP3FL) were 4 times more abundant during anti-FLAG immunoprecipitation, yet HA-NLRP3FL was still much less co-immunoprecipitated with FLAG-casp11C254A ΔSCAF compared to FLAG-casp11C254A FL. Immunoblotted proteins are indicated to left of each immunoblot panel. d, Schematic representation of CARD, DED, CASPASE p20 and CASPASE p10 domains of murine inflammatory and apoptotic caspases. SCAF domain is composed of CASPASE p20 and CASPASE p10 domains. All caspases, with the exception of the short forms of mouse and human caspase-12, have SCAF domains. The alignment E values were calculated using the NCBI alignment tool. e, Similarity coefficients between caspase-11 and other murine caspases. f, Protein sequence alignment for murine caspases. The alignment diagram was generated using the alignment module of SnapGene software. g, Schematic indicating the different caspase-11C254A and caspase-9 chimeras used for co-immunoprecipitation experiments in 293 T cells. All forms were fused to 3xFLAG tag in N-terminus.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed Western Blots.
Source Data Fig. 3
Unprocessed Western Blots.
Source Data Fig. 4
Unprocessed Western Blots.
Source Data Fig. 6
Unprocessed Western Blots.
Rights and permissions
About this article
Cite this article
Moretti, J., Jia, B., Hutchins, Z. et al. Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome. Nat Immunol 23, 705–717 (2022). https://doi.org/10.1038/s41590-022-01192-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01192-4
This article is cited by
-
Dectin-1 aggravates neutrophil inflammation through caspase-11/4-mediated macrophage pyroptosis in asthma
Respiratory Research (2024)
-
Adipose triglyceride lipase suppresses noncanonical inflammasome by hydrolyzing LPS
Nature Chemical Biology (2024)
-
The NLRP3 inflammasome: contributions to inflammation-related diseases
Cellular & Molecular Biology Letters (2023)
-
Targeting NLRP3 inflammasome for neurodegenerative disorders
Molecular Psychiatry (2023)
-
Pyroptosis in bone loss
Apoptosis (2023)