Abstract
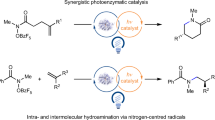
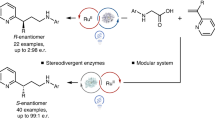
Enzymes are recognized as exceptional catalysts for achieving high stereoselectivities1,2,3, but their ability to control the reactivity and stereoinduction of free radicals lags behind that of chemical catalysts4. Thiamine diphosphate (ThDP)-dependent enzymes5 are well-characterized systems that inspired the development of N-heterocyclic carbenes (NHCs)6,7,8 but have not yet been proved viable in asymmetric radical transformations. There is a lack of a biocompatible and general radical-generation mechanism, as nature prefers to avoid radicals that may be harmful to biological systems9. Here we repurpose a ThDP-dependent lyase as a stereoselective radical acyl transferase (RAT) through protein engineering and combination with organophotoredox catalysis10. Enzyme-bound ThDP-derived ketyl radicals are selectively generated through single-electron oxidation by a photoexcited organic dye and then cross-coupled with prochiral alkyl radicals with high enantioselectivity. Diverse chiral ketones are prepared from aldehydes and redox-active esters (35 examples, up to 97% enantiomeric excess (e.e.)) by this method. Mechanistic studies reveal that this previously elusive dual-enzyme catalysis/photocatalysis directs radicals with the unique ThDP cofactor and evolvable active site. This work not only expands the repertoire of biocatalysis but also provides a unique strategy for controlling radicals with enzymes, complementing existing chemical tools.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials and from the Cambridge Crystallographic Data Centre (CCDC; https://www.ccdc.cam.ac.uk/structures/); crystallographic data are available free of charge under CCDC reference numbers 2256735 (3e) and 2256734 (3g).
References
Bell, E. L. et al. Biocatalysis. Nat. Rev. Methods Primers 1, 46 (2021).
Reetz, M. T. Biocatalysis in organic chemistry and biotechnology: past, present, and future. J. Am. Chem. Soc. 135, 12480–12496 (2013).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Hailes, H. C. et al. Engineering stereoselectivity of ThDP-dependent enzymes. FEBS J. 280, 6374–6394 (2013).
Enders, D., Niemeier, O. & Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 107, 5606–5655 (2007).
Bellotti, P., Koy, M., Hopkinson, M. N. & Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 5, 711–725 (2021).
Liu, K., Schwenzer, M. & Studer, A. Radical NHC catalysis. ACS Catal. 12, 11984–11999 (2022).
Högbom, M., Sjöberg, B.-M. & Berggren, G. in Encyclopedia of Life Sciences 375–393 (Wiley, 2020).
Stephenson, C. R., Yoon, T. P. & MacMillan, D. W. C. (eds) Visible Light Photocatalysis in Organic Chemistry (Wiley, 2018).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Studer, A. & Curran, D. P. Catalysis of radical reactions: a radical chemistry perspective. Angew. Chem. Int. Ed. 55, 58–102 (2016).
Zhang, C., Li, Z.-L., Gu, Q.-S. & Liu, X.-Y. Catalytic enantioselective C(sp3)–H functionalization involving radical intermediates. Nat. Commun. 12, 475 (2021).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Chen, X. et al. Intramolecular stereoselective Stetter reaction catalyzed by benzaldehyde lyase. Angew. Chem. Int. Ed. 60, 9326–9329 (2021).
Chabrière, E. et al. Crystal structure of the free radical intermediate of pyruvate: ferredoxin oxidoreductase. Science 294, 2559–2563 (2001).
Liu, X. et al. A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2-reducing enzyme. Nat. Chem. 10, 1201–1206 (2018).
Trimble, J. S. et al. A designed photoenzyme for enantioselective [2+2] cycloadditions. Nature 611, 709–714 (2022).
Sun, N. et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes. Nature 611, 715–720 (2022).
Sorigue, D. et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 357, 903–907 (2017).
Zhang, W. et al. Photobiocatalytic synthesis of chiral secondary fatty alcohols from renewable unsaturated fatty acids. Nat. Commun. 11, 2258 (2020).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166–1169 (2019).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Peng, Y. et al. Photoinduced promiscuity of cyclohexanone monooxygenase for the enantioselective synthesis of α-fluoroketones. Angew. Chem. Int. Ed. 61, e202211199 (2022).
Duan, X. et al. A photoenzymatic strategy for radical-mediated stereoselective hydroalkylation with diazo compounds. Angew. Chem. Int. Ed. 62, e202214135 (2023).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Xu, J. et al. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters. J. Am. Chem. Soc. 141, 7934–7945 (2019).
Jin, S. et al. N-heterocyclic carbene-photocatalyzed tricomponent regioselective 1,2-diacylation of alkenes illuminates the mechanistic details of the electron donor–acceptor complex-mediated radical relay processes. ACS Catal. 12, 285–294 (2021).
Delfau, L. et al. Critical assessment of the reducing ability of Breslow-type derivatives and implications for carbene-catalyzed radical reactions. Angew. Chem. Int. Ed. 60, 26783–26789 (2021).
Mulks, F. F., Melaimi, M., Yan, X., Baik, M. H. & Bertrand, G. How to enhance the efficiency of Breslow intermediates for SET catalysis. J. Org. Chem. 88, 2535–2542 (2023).
Hari, D. P. & König, B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 50, 6688–6699 (2014).
Schreiner, P. R. et al. Overcoming lability of extremely long alkane carbon–carbon bonds through dispersion forces. Nature 477, 308–311 (2011).
Acknowledgements
We thank N. Jiao, E. Meggers and H. Zhao for insightful discussions. S. Zhang, C. Zhang, J. Feng and B. Wang are appreciated for their help with mechanistic studies. We appreciate the financial support from the National Key Research and Development Program of China (2022YFA0913000 to X.H., 2019YFA0405600 to L.Y.), the National Natural Science Foundation of China (21825703 and 21927814 to C.T., 22277053 to X.H.), the Natural Science Foundation of Jiangsu Province (BK20220760 to X.H., BK20230018 to Y.L.), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB37000000 to C.T.), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2022455 to L.Y.) and Fundamental Research Funds for the Central Universities (020514380253 to Y.L.). Part of this work was performed at the Steady High Magnetic Field Facilities, High Magnetic Field Laboratory, Chinese Academy of Sciences. All theoretical calculations were performed at the High Performance Computing Center (HPCC) of Nanjing University.
Author information
Authors and Affiliations
Contributions
Y.X. developed the photocatalytic/biocatalytic system and performed most of the experiments. X.P., J.Z., Z.X. and Y.B. assisted in synthetic experiments and mechanistic investigations. H.C. and Y.L. performed theoretical calculations. L.Y. and A.L. carried out EPR measurements and analysis under the supervision of C.T. Y.Z. performed X-ray crystal-structure analysis. X.H. and Y.X. wrote the manuscript, with input from all authors. X.H. coordinated and conceived the project.
Corresponding authors
Ethics declarations
Competing interests
X.H., Y.X., X.P., J.Z., Z.X. and Y.B. are the inventors of a patent application submitted by Nanjing University that covers the photoenzymatic approach to chiral ketones under application number 202310939697.8. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Chen, H., Yu, L. et al. A light-driven enzymatic enantioselective radical acylation. Nature 625, 74–78 (2024). https://doi.org/10.1038/s41586-023-06822-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06822-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.