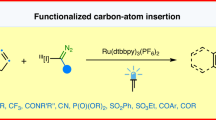
Abstract
When searching for the ideal molecule to fill a particular functional role (for example, a medicine), the difference between success and failure can often come down to a single atom1. Replacing an aromatic carbon atom with a nitrogen atom would be enabling in the discovery of potential medicines2, but only indirect means exist to make such C-to-N transmutations, typically by parallel synthesis3. Here, we report a transformation that enables the direct conversion of a heteroaromatic carbon atom into a nitrogen atom, turning quinolines into quinazolines. Oxidative restructuring of the parent azaarene gives a ring-opened intermediate bearing electrophilic sites primed for ring reclosure and expulsion of a carbon-based leaving group. Such a ‘sticky end’ approach subverts existing atom insertion–deletion approaches and as a result avoids skeleton-rotation and substituent-perturbation pitfalls common in stepwise skeletal editing. We show a broad scope of quinolines and related azaarenes, all of which can be converted into the corresponding quinazolines by replacement of the C3 carbon with a nitrogen atom. Mechanistic experiments support the critical role of the activated intermediate and indicate a more general strategy for the development of C-to-N transmutation reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are in the Supplementary Information or are available from the corresponding author upon reasonable request.
References
Boger, D. L. The difference a single atom can make: synthesis and design at the chemistry–biology interface. J. Org. Chem. 82, 11961–11980 (2017).
Pennington, L. D., Collier, P. N. & Comer, E. Harnessing the necessary nitrogen atom in chemical biology and drug discovery. Med. Chem. Res. https://doi.org/10.1007/s00044-023-03073-3 (2023).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Schönherr, H. & Cernak, T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew. Chem. Int. Ed. 52, 12256–12267 (2013).
Chiodi, D. & Ishihara, Y. “Magic chloro”: profound effects of the chlorine atom in drug discovery. J. Med. Chem. 66, 5305–5331 (2023).
Pennington, L. D. & Moustakas, D. T. The necessary nitrogen atom: a versatile high-impact design element for multiparameter optimization. J. Med. Chem. 60, 3552–3579 (2017).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Boss, C., Bolli, M. H. & Gatfield, J. From bosentan (Tracleer®) to macitentan (Opsumit®): the medicinal chemistry perspective. Bioorg. Med. Chem. Lett. 26, 3381–3394 (2016).
Eckhardt, M., Klein, T., Nar, H. & Thiemann, S. in Successful Drug Discovery (eds Fischer, J. & Rotella, D. P.) 129–156 (Wiley, 2015); https://doi.org/10.1002/9783527678433.ch7
Yamada, K., Sakamoto, T., Omori, K. & Kikkawa, K. in Successful Drug Discovery (eds Fischer, J. & Rotella, D. P.) 61–86 (Wiley, 2015); https://doi.org/10.1002/9783527678433.ch4
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Kelley, B. T., Walters, J. C. & Wengryniuk, S. E. Access to diverse oxygen heterocycles via oxidative rearrangement of benzylic tertiary alcohols. Org. Lett. 18, 1896–1899 (2016).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018).
Siddiqi, Z., Wertjes, W. C. & Sarlah, D. Chemical equivalent of arene monooxygenases: dearomative synthesis of arene oxides and oxepines. J. Am. Chem. Soc. 142, 10125–10131 (2020).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Lyu, H., Kevlishvili, I., Yu, X., Liu, P. & Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Morofuji, T., Inagawa, K. & Kano, N. Sequential ring-opening and ring-closing reactions for converting para-substituted pyridines into meta-substituted anilines. Org. Lett. 23, 6126–6130 (2021).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Wang, J., Lu, H., He, Y., Jing, C. & Wei, H. Cobalt-catalyzed nitrogen atom insertion in arylcycloalkenes. J. Am. Chem. Soc. 144, 22433–22439 (2022).
Kamitani, M. et al. Single–carbon atom transfer to α,β-unsaturated amides from N-heterocyclic carbenes. Science 379, 484–488 (2023).
Woo, J. et al. Scaffold hopping by net photochemical carbon deletion of azaarenes. Science 376, 527–532 (2022).
Liu, S. & Cheng, X. Insertion of ammonia into alkenes to build aromatic N-heterocycles. Nat. Commun. 13, 425 (2022).
Sundberg, R. J., Suter, S. R. & Brenner, M. Photolysis of 0-substituted aryl azides in diethylamine. Formation and autoxidation of 2-diethylamino-1H-azepine intermediates. J. Am. Chem. Soc. 94, 513–520 (1972).
Patel, S. C. & Burns, N. Z. Conversion of aryl azides to aminopyridines. J. Am. Chem. Soc. 144, 17797–17802 (2022).
Chen, P., Billett, B. A., Tsukamoto, T. & Dong, G. ‘Cut and sew’ transformations via transition-metal-catalyzed carbon–carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Boyle, B. T., Levy, J. N., de Lescure, L., Paton, R. S. & McNally, A. Halogenation of the 3-position of pyridines through Zincke imine intermediates. Science 378, 773–779 (2022).
Loenen, W. A. M., Dryden, D. T. F., Raleigh, E. A., Wilson, G. G. & Murray, N. E. Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res. 42, 3–19 (2014).
Fisher, T. J. & Dussault, P. H. Alkene ozonolysis. Tetrahedron 73, 4233–4258 (2017).
Smaligo, A. J. et al. Hydrodealkenylative C(sp3)–C(sp2) bond fragmentation. Science 364, 681–685 (2019).
Fremery, M. I. & Fields, E. K. Amozonolysis of cycloolefins. J. Org. Chem. 29, 2240–2243 (1964).
Willand-Charnley, R., Fisher, T. J., Johnson, B. M. & Dussault, P. H. Pyridine Is an organocatalyst for the reductive ozonolysis of alkenes. Org. Lett. 14, 2242–2245 (2012).
An, W. et al. Site-selective C8-alkylation of quinoline N-oxides with maleimides under Rh(III) catalysis. J. Org. Chem. 86, 7579–7587 (2021).
Hwang, H., Kim, J., Jeong, J. & Chang, S. Regioselective introduction of heteroatoms at the C-8 position of quinoline N-oxides: remote C–H activation using N-oxide as a stepping stone. J. Am. Chem. Soc. 136, 10770–10776 (2014).
Chen, X., Cui, X. & Wu, Y. C8-selective acylation of quinoline N-oxides with α-oxocarboxylic acids via palladium-catalyzed regioselective C–H bond activation. Org. Lett. 18, 3722–3725 (2016).
Albini, A. & Alpegiani, M. The photochemistry of the N-oxide function. Chem. Rev. 84, 43–71 (1984).
Spence, G. G., Taylor, E. C. & Buchardt, O. Photochemical reactions of azoxy compounds, nitrones, and aromatic amine N-oxides. Chem. Rev. 70, 231–265 (1970).
Hurlow, E. E. et al. Photorearrangement of [8]-2,6-pyridinophane N-oxide. J. Am. Chem. Soc. 142, 20717–20724 (2020).
Shieh, P., Hill, M. R., Zhang, W., Kristufek, S. L. & Johnson, J. A. Clip chemistry: diverse (bio)(macro)molecular and material function through breaking covalent bonds. Chem. Rev. 121, 7059–7121 (2021).
Cochran, B. M. et al. Development of a commercial process to prepare AMG 232 using a green ozonolysis–Pinnick tandem transformation. J. Org. Chem. 84, 4763–4779 (2019).
Ragan, J. A. et al. Safe execution of a large-scale ozonolysis: preparation of the bisulfite adduct of 2-hydroxyindan-2-carboxaldehyde and its utility in a reductive amination. Org. Process Res. Dev. 7, 155–160 (2003).
Van Ornum, S. G., Champeau, R. M. & Pariza, R. Ozonolysis applications in drug synthesis. Chem. Rev. 106, 2990–3001 (2006).
Blair, H. A. Belumosudil: first approval. Drugs 81, 1677–1682 (2021).
Malherbe, P. et al. Me-Talnetant and Osanetant interact within overlapping but not identical binding pockets in the human tachykinin neurokinin 3 receptor transmembrane domains. Mol. Pharmacol. 73, 1736–1750 (2008).
Dexter, D. L. et al. Activity of a novel 4-quinolinecarboxylic acid, NSC 368390 [6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt], against experimental tumors.Cancer Res. 45, 5563–5568 (1985).
Ruffoni, A., Hampton, C., Simonetti, M. & Leonori, D. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature 610, 81–86 (2022).
Wise, D. E. et al. Photoinduced oxygen transfer using nitroarenes for the anaerobic cleavage of alkenes. J. Am. Chem. Soc. 144, 15437–15442 (2022).
Griesbaum, K. et al. Ozonolysis of vinyl ethers in solution and on polyethylene. J. Org. Chem. 55, 6153–6161 (1990).
Wojciechowski, B. J., Chiang, C. Y. & Kuczkowski, R. L. Ozonolysis of 1,1-dimethoxyethene, 1,2-dimethoxyethene and vinyl acetate. J. Org. Chem. 55, 1120–1122 (1990).
Ko, S., Na, Y. & Chang, S. A novel chelation-assisted hydroesterification of alkenes via ruthenium catalysis. J. Am. Chem. Soc. 124, 750–751 (2002).
Gollnick, K. & Koegler, S. Thermal transformations of oxazole endoperoxides: rearrangements, fragmentations and methanol additions. Tetrahedron Lett. 29, 1007–1010 (1988).
Gobec, S. et al. in Science of Synthesis (eds Yamamoto, Y. & Shinkai, I.) 573–750 (Thieme, 2004); https://doi.org/10.1055/sos-SD-016-00745
Kohlmeyer, C., Schäfer, A., Huy, P. H. & Hilt, G. Formamide-catalyzed nucleophilic substitutions: mechanistic insight and rationalization of catalytic activity. ACS Catal. 10, 11567–11577 (2020).
Acknowledgements
We thank the Rawal, Snyder and Dong groups (University of Chicago) for lending chemicals. We thank the Snyder group for use of an ozone generator and the Rawal group for use of a cryocooler. We thank T. Pearson (University of Chicago), A. Neel (Merck) and J. Del Pozo (Merck) for helpful discussions. Financial support for this work was provided by the National Institutes of Health (grant no. R35 GM142768).
Author information
Authors and Affiliations
Contributions
J.W. and C.S. designed and conducted experiments, and collected and analysed the data. M.D.L. and J.W. conceived of the project and wrote the manuscript with input from all authors. M.D.L. and A.H.C. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Mattia Silvi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods; general procedure for transmutation; graphical guide; general procedure for N-oxidation of azaarenes; starting material synthesis; one-pot transmutation; synthetic applications; mechanistic studies; optimization; limitations; references; and nuclear magnetic resonance spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woo, J., Stein, C., Christian, A.H. et al. Carbon-to-nitrogen single-atom transmutation of azaarenes. Nature 623, 77–82 (2023). https://doi.org/10.1038/s41586-023-06613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06613-4
This article is cited by
-
Atom-swap chemistry could aid drug discovery
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.