Abstract
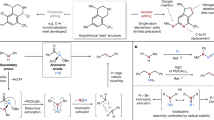
Skeletal editing has received unprecedented attention as an emerging technology for the late-stage manipulation of molecular scaffolds. The direct achievement of functionalized carbon-atom insertion in aromatic rings is challenging. Despite ring-expanding carbon-atom insertion reactions, such as the Ciamician–Dennstedt re-arrangement, being performed for more than 140 years, only a few relevant examples of such transformations have been reported, with these limited to the installation of halogen, ester and phenyl groups. Here we describe a photoredox-enabled functionalized carbon-atom insertion reaction into indene. We disclose the utilization of a radical carbyne precursor that facilitates the insertion of carbon atoms bearing a variety of functional groups, including trifluoromethyl, ester, phosphate ester, sulfonate ester, sulfone, nitrile, amide, aryl ketone and aliphatic ketone fragments to access a library of 2-substituted naphthalenes. The application of this methodology to the skeletal editing of molecules of pharmaceutical relevance highlights its utility.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the main text and its Supplementary Information file. Source data are provided as Source Data file. Data are also available from the corresponding author upon request. Crystallographic information data files and xyz coordinates of the optimized structures are available as supplementary files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition no. CCDC 2262556 (3y). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Haut, F. L. et al. Synthesis of pyrroles via consecutive 6π-electrocyclization/ring-contraction of sulfilimines. J. Am. Chem. Soc. 143, 9002–9008 (2021).
Morofuji, T., Nagai, S., Watanabe, A., Inagawa, K. & Kano, N. Streptocyanine as an activation mode of amine catalysis for the conversion of pyridine rings to benzene rings. Chem. Sci. 14, 485–490 (2023).
Joynson, B. W. & Ball, L. T. Skeletal editing: interconversion of arenes and heteroarenes. Helv. Chim. Acta 106, e202200182 (2023).
Hui, C., Wang, Z., Wang, S. & Xu, C. Molecular editing in natural product synthesis. Org. Chem. Front. 9, 1451–1457 (2022).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Woo, J. et al. Scaffold hopping by net photochemical carbon deletion of azaarenes. Science 376, 527–532 (2022).
Lyu, H., Kevlishvili, I., Yu, X., Liu, P. & Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Patel, S. C. & Burns, N. Z. Conversion of aryl azides to aminopyridines. J. Am. Chem. Soc. 144, 17797–17802 (2022).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Liu, S. & Cheng, X. Insertion of ammonia into alkenes to build aromatic N-heterocycles. Nat. Commun. 13, 425 (2022).
Kelly, P. Q., Filatov, A. S. & Levin, M. D. A synthetic cycle for heteroarene synthesis by nitride insertion. Angew. Chem. Int. Ed. 61, e202213041 (2022).
Finkelstein, P. et al. Nitrogen atom insertion into indenes to access isoquinolines. Chem. Sci. 14, 2954–2959 (2023).
Morofuji, T., Kinoshita, H. & Kano, N. Connecting a carbonyl and a pi-conjugated group through a p-phenylene linker by (5+1) benzene ring formation. Chem. Commun. 55, 8575–8578 (2019).
Sattler, A. & Parkin, G. Cleaving carbon–carbon bonds by inserting tungsten into unstrained aromatic rings. Nature 463, 523–526 (2010).
Ciamician, G. L. & Dennstedt, M. Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols. Ber. Dtsch. Chem. Ges. 14, 1153–1163 (1881).
Ma, D., Martin, B. S., Gallagher, K. S., Saito, T. & Dai, M. One-carbon insertion and polarity inversion enabled a pyrrole strategy to the total syntheses of pyridine-containing lycopodium alkaloids: complanadine a and lycodine. J. Am. Chem. Soc. 143, 16383–16387 (2021).
Wynberg, H. The Reimer–Tiemann reaction. Chem. Rev. 60, 169–184 (1960).
Morten, M., Hennum, M. & Bonge-Hansen, T. Synthesis of quinoline-3-carboxylates by a Rh(II)-catalyzed cyclopropanation–ring expansion reaction of indoles with halodiazoacetates. Beilstein J. Org. Chem. 11, 1944–1949 (2015).
Dherange, B. D., Kelly, P. Q., Liles, J. P., Sigman, M. S. & Levin, M. D. Carbon atom insertion into pyrroles and indoles promoted by chlorodiazirines. J. Am. Chem. Soc. 143, 11337–11344 (2021).
Hyland, E. E., Kelly, P. Q., McKillop, A. M., Dherange, B. D. & Levin, M. D. Unified access to pyrimidines and quinazolines enabled by N–N cleaving carbon atom insertion. J. Am. Chem. Soc. 144, 19258–19264 (2022).
Gabriele, B., Mancuso, R. & Veltri, L. Recent advances in the synthesis of indanes and indenes. Chem. Eur. J. 22, 5056–5094 (2016).
Prasher, P. & Sharma, M. Medicinal chemistry of indane and its analogues: a mini review. ChemistrySelect 6, 2658–2677 (2021).
Unzner, T. A., Grossmann, A. S. & Magauer, T. Rapid access to orthogonally functionalized naphthalenes: application to the total synthesis of the anticancer agent chartarin. Angew. Chem. Int. Ed. 55, 9763–9767 (2016).
Banerjee, S. et al. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 42, 1601–1618 (2013).
Parham, W. E. & Reiff, H. E. Ring expansion during the reaction of indenylsodium and chloroform. J. Am. Chem. Soc. 77, 1177–1178 (1955).
Parham, W. E., Reiff, H. E. & Swartzentruhe, P. The formation of naphthalenes from indenes. J. Am. Chem. Soc. 78, 1437–1440 (1956).
Weiss, R., Seubert, J. & Hampel, F. α-Aryliodonio diazo compounds: SN reactions at the α-C atom as a novel reaction type for diazo compounds. Angew. Chem. Int. Ed. 33, 1952–1953 (1994).
Wang, Z., Herraiz, A. G., Del Hoyo, A. M. & Suero, M. G. Generating carbyne equivalents with photoredox catalysis. Nature 554, 86–91 (2018).
Wang, X. et al. Convergent synthesis of 1,4-dicarbonyl Z-alkenes through three-component coupling of alkynes, α-diazo sulfonium triflate, and water. J. Am. Chem. Soc. 144, 4952–4965 (2022).
Ansari, M. A., Kumar, G. & Singh, M. S. Base mediated diazirination via iodine(III) reagents. Org. Lett. 24, 2815–2820 (2022).
Jiang, L., Wang, Z., Armstrong, M. & Suero, M. G. β-Diazocarbonyl compounds: synthesis and their Rh(II)-catalyzed 1,3 C–H insertions. Angew. Chem. Int. Ed. 60, 6177–6184 (2021).
Taylor, M. T., Nelson, J. E., Suero, M. G. & Gaunt, M. J. A protein functionalization platform based on selective reactions at methionine residues. Nature 562, 563–568 (2018).
Tu, H. F., Jeandin, A. & Suero, M. G. catalytic synthesis of cyclopropenium cations with Rh-carbynoids. J. Am. Chem. Soc. 144, 16737–16743 (2022).
Wang, Z., Jiang, L., Sarro, P. & Suero, M. G. Catalytic cleavage of C(sp2)–C(sp2) bonds with Rh-carbynoids. J. Am. Chem. Soc. 141, 15509–15514 (2019).
Palomo, E. et al. Generating Fischer-type Rh-carbenes with Rh-carbynoids. J. Am. Chem. Soc. 145, 4975–4981 (2023).
Bonge, H. T., Pintea, B. & Hansen, T. Highly efficient formation of halodiazoacetates and their use in stereoselective synthesis of halocyclopropanes. Org. Biomol. Chem. 6, 3670–3672 (2008).
Li, X., Golz, C. & Alcarazo, M. α-Diazo sulfonium triflates: synthesis, structure, and application to the synthesis of 1-(dialkylamino)-1,2,3-triazoles. Angew. Chem. Int. Ed. 60, 6943–6948 (2021).
Schnaars, C., Hennum, M. & Bonge-Hansen, T. Nucleophilic halogenations of diazo compounds, a complementary principle for the synthesis of halodiazo compounds: experimental and theoretical studies. J. Org. Chem. 78, 7488–7497 (2013).
Mejía, E. & Togni, A. Rhenium-catalyzed trifluoromethylation of arenes and heteroarenes by hypervalent iodine reagents. ACS Catal. 2, 521–527 (2012).
Keaveney, S. T. & Schoenebeck, F. Palladium-catalyzed decarbonylative trifluoromethylation of acid fluorides. Angew. Chem. Int. Ed. 57, 4073–4077 (2018).
Shroot, B. & Michel, S. Pharmacology and chemistry of adapalene. J. Am. Acad. Dermatol. 97, 96–103 (1997).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Acknowledgements
Generous financial support by the Deutsche Forschungsgemeinschaft (Leibniz Award) and the Alexander von Humboldt Foundation (C.C.C. and J.T.) is gratefully acknowledged. O.G. gratefully acknowledges financial support from the National Institutes of Health (R35GM137797), the Camille and Henry Dreyfus Foundation and the Welch Foundation (A-2102-20220331) for supporting this work. O.G. also acknowledges the Texas A&M University HPRC resources (https://hprc.tamu.edu) for computational resources. We sincerely thank G. Tan, H. Wang, R. Kleinmans and A. Heusler for help in preparing the manuscript and many helpful discussions.
Author information
Authors and Affiliations
Contributions
F.G. and F.-P.W. conceived the project. F.-P.W. performed the initial screening experiments. F.-P.W. and C.C.C. performed synthetic experiments. R.L. and P.M. conducted computations. C.G.D. analysed X-ray structures. F.-P.W. and F.G. supervised research. F.-P.W., J.T. and F.G. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Lei Shi, Andrius Merkys and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, note, Figs. 1–265 and Tables 1–4.
Supplementary Data 1
Cartesian coordinates for all calculated structures.
Supplementary Data 2
Crystallographic data of compound 3y.
Supplementary Data 3
CheckCIF data of compound 3y.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, FP., Chintawar, C.C., Lalisse, R. et al. Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion. Nat Catal 7, 242–251 (2024). https://doi.org/10.1038/s41929-023-01089-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01089-x
This article is cited by
-
Drug design via single-carbon atom insertion
Nature Catalysis (2024)