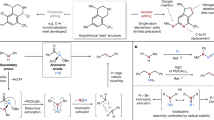
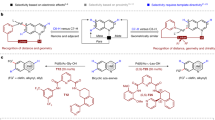
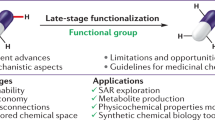
Abstract
Medicinal chemistry continues to be impacted by new synthetic methods. Particularly sought after, especially at the drug discovery stage, is the ability to enact the desired chemical transformations in a concise and chemospecific fashion. To this end, the field of organic synthesis has become captivated by the idea of ‘molecular editing’—to rapidly build onto, change or prune molecules one atom at a time using transformations that are mild and selective enough to be employed at the late stages of a synthetic sequence. In this Review, the definition and categorization of a particularly promising subclass of molecular editing reactions, termed ‘single-atom skeletal editing’, are proposed. Although skeletal editing applies to both cyclic and acyclic compounds, this Review focuses on heterocycles, both for their centrality in medicinal chemistry and for the definitional clarity afforded by a focus on ring systems. A classification system is presented by highlighting methods (both historically important examples and recent advances) that achieve such transformations, with the goal to spark interest and inspire further development in this growing field.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Hann, M. M. & Keserü, G. M. Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nat. Rev. Drug Discov. 11, 355–365 (2012).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in Drugs. J. Med. Chem. 57, 5845–5859 (2014).
Majumdar, K. & Chattopadhyay, S. Heterocycles in Natural Product Synthesis (John Wiley & Sons, Ltd, 2011).
Antermite, D. & Bull, J. A. Transition metal-catalyzed directed C(sp3)–H functionalization of saturated heterocycles. Synthesis 51, 3171–3204 (2019).
Campos, K. R. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 36, 1069–1084 (2007).
Ogba, O. M., Warner, N. C., O’Leary, D. J. & Grubbs, R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 47, 4510–4544 (2018).
Albright, H. et al. Carbonyl–olefin metathesis. Chem. Rev. 121, 9359–9406 (2021).
Wang, B., Perea, M. A. & Sarpong, R. Transition metal-mediated C−C single bond cleavage: making the cut in total synthesis. Angew. Chem. Int. Ed. 59, 18898–18919 (2020).
Engle, K. M. et al. in Organic Chemistry – Breakthroughs and Perspectives (eds Ding, K. & Dai, L.-X.) 279–333 (John Wiley & Sons, Ltd, 2012).
Bhawal, B. N. & Morandi, B. Shuttle catalysis—new strategies in organic synthesis. Chem. Eur. J. 23, 12004–12013 (2017).
Qiu, X. et al. Cleaving arene rings for acyclic alkenylnitrile synthesis. Nature 597, 64–69 (2021).
Aube, J. & Milligan, G. L. Intramolecular Schmidt reaction of alkyl azides. J. Am. Chem. Soc. 113, 8965–8966 (1991).
Silva, L. F. Construction of cyclopentyl units by ring contraction reactions. Tetrahedron 58, 9137–9161 (2002).
Harmata, M. in Molecular Rearrangements in Organic Synthesis (ed. Rocas, C. M.) 183–226 (John Wiley & Sons, Ltd, 2015).
Furniss, B. S., Hannaford, A. J., Smith, P. W. G. & Tatchell, A. R. Vogel’s Textbook of Practical Organic Chemistry 5th edn, 1111–1114 (Longman, 1989).
Jamison, T. F., Shambayati, S., Crowe, W. E. & Schreiber, S. L. Tandem use of cobalt-mediated reactions to synthesize (+)-epoxydictymene, a diterpene containing a trans-fused 5−5 ring system. J. Am. Chem. Soc. 119, 4353–4363 (1997).
Wolinsky, J., Gibson, T., Chan, D. & Wolf, H. Stereospecific synthesis of iridomyrmecin and related iridolactones. Tetrahedron 21, 1247–1261 (1965).
Stoltz, B. M. & Wood, J. L. The stereoselective ring contraction of a pyranosylated indolocarbazole. A biosynthetic link between K252a and staurosporine? Tetrahedron Lett. 37, 3929–3930 (1996).
Xiao, M. et al. Total synthesis of (−)-isatisine A via a biomimetic benzilic acid rearrangement. Tetrahedron 71, 3705–3714 (2015).
Tehrani, K. A. et al. Boron(III) bromide-induced ring contraction of 3-oxygenated piperidines to 2-(bromomethyl)pyrrolidines. Tetrahedron Lett. 41, 2507–2510 (2000).
Feraldi-Xypolia, A., Gomez Pardo, D. & Cossy, J. Ring Contraction of 3-hydroxy-3-(trifluoromethyl)piperidines: synthesis of 2-substituted 2-(trifluoromethyl)pyrrolidines. Chem. Eur. J. 21, 12876–12880 (2015).
Álvarez-Dorta, D., León, E. I., Kennedy, A. R., Riesco-Fagundo, C. & Suárez, E. Sequential Norrish Type II photoelimination and intramolecular aldol cyclization of 1,2-diketones in carbohydrate systems: stereoselective synthesis of cyclopentitols. Angew. Chem. Int. Ed. 47, 8917–8919 (2008).
Alvarez-Dorta, D. et al. Sequential Norrish Type II photoelimination and intramolecular aldol cyclization of α-diketones: synthesis of polyhydroxylated cyclopentitols by ring contraction of hexopyranose carbohydrate derivatives. Chem. Eur. J. 19, 10312–10333 (2013).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Ling, R. & Mariano, P. S. A demonstration of the synthetic potential of pyridinium salt photochemistry by its application to a stereocontrolled synthesis of (+)-mannostatin A1. J. Org. Chem. 63, 6072–6076 (1998).
Wang, S., Wang, H., Tian, N. & Yan, H. Insights for diastereoselectivity in synthesis of 2,3-dihydropyrroles by photochemical ring contraction of 1,4-dihydropyridines. Tetrahedron Lett. 65, 152797 (2021).
Bellamy, F., Martz, P. & Streith, L. An intriguing copper salt effect upon the photochemistry of pyridine-N-oxides. Specific photoinduced syntheses of 3-substituted 2-formylpyrroles. Heterocycles 3, 395–400 (1975).
Weber, H. & Rohn, T. 2H-Azirine und 2-(ω-Cyanalkyl)furane als neuartige Photoprodukte aus [n](2,6)Pyridinophan-N-oxiden und ihre Bedeutung für den Reaktionsverlauf. Chem. Ber. 122, 945–950 (1989).
Feng, Z., Allred, T. K., Hurlow, E. E. & Harran, P. G. Anomalous chromophore disruption enables an eight-step synthesis and stereochemical reassignment of (+)-marineosin A. J. Am. Chem. Soc. 141, 2274–2278 (2019).
Hurlow, E. E. et al. Photorearrangement of [8]-2,6-pyridinophane N-oxide. J. Am. Chem. Soc. 142, 20717–20724 (2020).
Padwa, A., Smolanoff, J. & Tremper, A. Intramolecular photochemical and thermal cyclization reactions of 2-vinyl substituted 2H-azirines. Tetrahedron Lett. 15, 29–32 (1974).
Padwa, A., Smolanoff, J. & Tremper, A. Photochemical transformations of small ring heterocyclic systems. LXV. Intramolecular cycloaddition reactions of vinyl-substituted 2H-azirines. J. Am. Chem. Soc. 97, 4682–4691 (1975).
Portillo, M., Maxwell, M. A. & Frederich, J. H. Synthesis of nitrogen heterocycles via photochemical ring opening of pyridazine N-oxides. Org. Lett. 18, 5142–5145 (2016).
Eaton, P. E. & Cole, T. W. The cubane system. J. Am. Chem. Soc. 86, 962–964 (1964).
Eaton, P. E. & Cole, T. W. Cubane. J. Am. Chem. Soc. 86, 3157–3158 (1964).
Yu, X. et al. Stereospecific construction of contiguous quaternary all‐carbon centers by oxidative ring contraction. Angew. Chem. Int. Ed. 156, 350–353 (2017).
Nicolaou, K. C., Gray, D. L. F. & Tae, J. Total synthesis of hamigerans and analogues thereof. Photochemical generation and Diels−Alder trapping of hydroxy-o-quinodimethanes. J. Am. Chem. Soc. 126, 613–627 (2004).
Ng, D., Yang, Z. & Garcia-Garibay, M. A. Total synthesis of (±)-herbertenolide by stereospecific formation of vicinal quaternary centers in a crystalline ketone. Org. Lett. 6, 645–647 (2004).
Natarajan, A., Ng, D., Yang, Z. & Garcia-Garibay, M. A. Parallel syntheses of (+)- and (−)-α-cuparenone by radical combination in crystalline solids. Angew. Chem. Int. Ed. 46, 6485–6487 (2007).
Dotson, J. J., Bachman, J. L., Garcia-Garibay, M. A. & Garg, N. K. Discovery and total synthesis of a bis(cyclotryptamine) alkaloid bearing the elusive piperidinoindoline scaffold. J. Am. Chem. Soc. 142, 11685–11690 (2020).
Dotson, J. J. et al. Taming radical pairs in the crystalline solid state: discovery and total synthesis of psychotriadine. J. Am. Chem. Soc. 143, 4043–4054 (2021).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018).
Zippel, C., Seibert, J. & Bräse, S. Skeletal editing—nitrogen deletion of secondary amines by anomeric amide reagents. Angew. Chem. Int. Ed. 60, 19522–19524 (2021).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Qin, H. et al. N‐atom deletion in nitrogen heterocycles. Angew. Chem. Int. Ed. 60, 20678–20683 (2021).
Hui, C., Brieger, L., Strohmann, C. & Antonchick, A. P. Stereoselective synthesis of cyclobutanes by contraction of pyrrolidines. J. Am. Chem. Soc. 143, 18864–18870 (2021).
Cao, Z.-C. & Shi, Z.-J. Deoxygenation of ethers to form carbon–carbon bonds via nickel catalysis. J. Am. Chem. Soc. 139, 6546–6549 (2017).
Song, Z.-L., Fan, C.-A. & Tu, Y.-Q. Semipinacol rearrangement in natural product synthesis. Chem. Rev. 111, 7523–7556 (2011).
Dowd, P. & Choi, S. C. A new tributyltin hydride-based rearrangement of bromomethyl β-keto esters. A synthetically useful ring expansion to ɣ-keto esters. J. Am. Chem. Soc. 109, 3493–3494 (1987).
Beckwith, A. L. J., O’Shea, D. M. & Westwood, S. W. Rearrangement of suitably constituted aryl, alkyl, or vinyl radicals by acyl or cyano group migration. J. Am. Chem. Soc. 110, 2565–2575 (1988).
Fu, C. et al. Diastereoselective total synthesis of salvileucalin C. Org. Lett. 16, 3376–3379 (2014).
Chen, P., Sieber, J., Senanayake, C. H. & Dong, G. Rh-catalyzed reagent-free ring expansion of cyclobutenones and benzocyclobutenones. Chem. Sci. 6, 5440–5445 (2015).
Kelley, B. T., Walters, J. C. & Wengryniuk, S. E. Access to diverse oxygen heterocycles via oxidative rearrangement of benzylic tertiary alcohols. Org. Lett. 18, 1896–1899 (2016).
Murai, K., Komatsu, H., Nagao, R. & Fujioka, H. Oxidative rearrangement of spiro cyclobutane cyclic aminals: efficient construction of bicyclic amidines. Org. Lett. 14, 772–775 (2012).
Mailloux, M. J., Fleming, G. S., Kumta, S. S. & Beeler, A. B. Unified synthesis of azepines by visible-light-mediated dearomative ring expansion of aromatic N-ylides. Org. Lett. 23, 525–529 (2021).
Odum, R. A. & Brenner, M. Rearrangement on deoxygenation of nitrosobenzene. J. Am. Chem. Soc. 88, 2074–2075 (1966).
Wentrup, C. Flash vacuum pyrolysis of azides, triazoles, and tetrazoles. Chem. Rev. 117, 4562–4623 (2017).
Gritsan, N. P. et al. Ring-expansion reaction of cyano-substituted singlet phenyl nitrenes: theoretical predictions and kinetic results from laser flash photolysis and chemical trapping experiments. J. Am. Chem. Soc. 123, 1425–1433 (2001).
Inui, H., Sawada, K., Oishi, S., Ushida, K. & McMahon, R. J. Aryl nitrene rearrangements: spectroscopic observation of a benzazirine and its ring expansion to a ketenimine by heavy-atom tunneling. J. Am. Chem. Soc. 135, 10246–10249 (2013).
Li, G. et al. An improved PIII/PV=O-catalyzed reductive C–N coupling of nitroaromatics and boronic acids by mechanistic differentiation of rate- and product-determining steps. J. Am. Chem. Soc. 142, 6786–6799 (2020).
Beckmann, E. Zur Kenntniss der Isonitrosoverbindungen. Ber. Dtsch. Chem. Ges. 19, 988–993 (1886).
Tinge, J. et al. in Ullmann’s Encyclopedia of Industrial Chemistry (American Cancer Society, 2018); https://doi.org/10.1002/14356007.a05_031.pub3
Banić Tomišić, Z. The story of azithromycin. Kem. Ind. 60, 603–617 (2011).
Amice, P., Blanco, L. & Conia, J. M. Enol silyl ethers and their use for the synthesis of α-halo-α,β-unsaturated carbonyl compounds. Synthesis 1976, 196–197 (1976).
Parham, W. E., Soeder, R. W., Throckmorton, J. R., Kuncl, K. & Dodson, R. M. Reactions of enol ethers with carbenes. V. Rearrangements of dihalocyclopropanes derived from six-, seven-, and eight-membered cyclic enol ethers. J. Am. Chem. Soc. 87, 321–328 (1965).
Skattebøl, L. Chemistry of gem-dihalocyclopropanes. IV. Ring opening of gem-dichlorocyclopropyl ethers. J. Org. Chem. 31, 1554–1559 (1966).
Ohno, M. A new ring-expansion reaction from enamine and dichlorocarbene. Tetrahedron Lett. 4, 1753–1755 (1963).
De Selms, R. C. A new method of ring expansion. Tetrahedron Lett. 7, 1965–1968 (1966).
Yuan, P., Gerlinger, C. K. G., Herberger, J. & Gaich, T. Ten-step asymmetric total synthesis of (+)-pepluanol A. J. Am. Chem. Soc. 143, 11934–11938 (2021).
Ciamician, G. L. & Dennstedt, M. Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols. Ber. Dtsch. Chem. Ges. 14, 1153–1163 (1881).
Wynberg, H. The Reimer–Tiemann reaction. Chem. Rev. 60, 169–184 (1960).
Dherange, B. D., Kelly, P. Q., Liles, J. P., Sigman, M. S. & Levin, M. D. Carbon atom insertion into pyrroles and indoles promoted by chlorodiazirines. J. Am. Chem. Soc. 143, 11337–11344 (2021).
Graham, W. H. The halogenation of amidines. I. Synthesis of 3-halo- and other negatively substituted diazirines. J. Am. Chem. Soc. 87, 4396–4397 (1965).
Ma, D., Martin, B. S., Gallagher, K. S., Saito, T. & Dai, M. One-carbon insertion and polarity inversion enabled a pyrrole strategy to the total syntheses of pyridine-containing lycopodium alkaloids: complanadine A and lycodine. J. Am. Chem. Soc. 143, 16383–16387 (2021).
Lyu, H., Kevlishvili, I., Yu, X., Liu, P. & Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Pennington, L. D. & Moustakas, D. T. The necessary nitrogen atom: a versatile high-impact design element for multiparameter optimization. J. Med. Chem. 60, 3552–3579 (2017).
Golding, B. T. in Successful Drug Discovery (eds Fischer, J., Klein, C. & Childers, W. E.) 201–223 (John Wiley & Sons, Ltd, 2019).
Fout, A. R., Bailey, B. C., Tomaszewski, J. & Mindiola, D. J. Cyclic denitrogenation of N-heterocycles applying a homogeneous titanium reagent. J. Am. Chem. Soc. 129, 12640–12641 (2007).
Morofuji, T., Inagawa, K. & Kano, N. Sequential ring-opening and ring-closing reactions for converting para-substituted pyridines into meta-substituted anilines. Org. Lett. 23, 6126–6130 (2021).
Acknowledgements
R.S. is grateful to the National Institutes of Medical Science (R35 GM13045) and Merck Research Laboratories for funding portions of the work described herein. M.D.L. thanks the Packard Foundation and National Institutes of Health (R35 GM142768) for funding. S.F.K. is grateful for a National Science Foundation Fellowship.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the inception, organization and writing of this manuscript, which was overseen by R.S. and M.D.L. All the figures were finalized by J.J. from initial drafts created by all the co-workers. J.J. led the writing of the section Contractions, S.F.K. wrote the section Deletions, J.W. led the writing of the section Expansions and B.D.D. wrote the Insertions section.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Peter Seavill was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jurczyk, J., Woo, J., Kim, S.F. et al. Single-atom logic for heterocycle editing. Nat. Synth 1, 352–364 (2022). https://doi.org/10.1038/s44160-022-00052-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00052-1
This article is cited by
-
Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion
Nature Catalysis (2024)
-
Skeletal editing of pyridines through atom-pair swap from CN to CC
Nature Chemistry (2024)
-
Drug design via single-carbon atom insertion
Nature Catalysis (2024)
-
Tunable molecular editing of indoles with fluoroalkyl carbenes
Nature Chemistry (2024)
-
A catalytic process enables efficient and programmable access to precisely altered indole alkaloid scaffolds
Nature Chemistry (2024)