Abstract
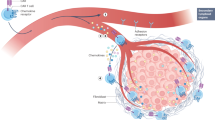
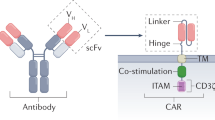
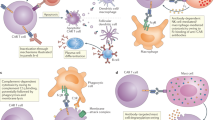
Engineering a patient’s own T cells to selectively target and eliminate tumour cells has cured patients with untreatable haematologic cancers. These results have energized the field to apply chimaeric antigen receptor (CAR) T therapy throughout oncology. However, evidence from clinical and preclinical studies underscores the potential of CAR T therapy beyond oncology in treating autoimmunity, chronic infections, cardiac fibrosis, senescence-associated disease and other conditions. Concurrently, the deployment of new technologies and platforms provides further opportunity for the application of CAR T therapy to noncancerous pathologies. Here we review the rationale behind CAR T therapy, current challenges faced in oncology, a synopsis of preliminary reports in noncancerous diseases, and a discussion of relevant emerging technologies. We examine potential applications for this therapy in a wide range of contexts. Last, we highlight concerns regarding specificity and safety and outline the path forward for CAR T therapy beyond cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Irvine, D. J., Maus, M. V., Mooney, D. J. & Wong, W. W. The future of engineered immune cell therapies. Science 378, 853–858 (2022).
Finck, A. V., Blanchard, T., Roselle, C. P., Golinelli, G. & June, C. H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 28, 678–689 (2022).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Mitsuyasu, R. T. et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus–infected subjects. Blood 96, 785–793 (2000). The first clinical trial deploying CAR T cells in HIV.
Roberts, M. R. et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 84, 2878–2889 (1994).
Levine, B. L. et al. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science 272, 1939–1943 (1996).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Grupp, S. A. et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Brudno, J. N. et al. T cells genetically modified to express an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 36, 2267–2280 (2018).
Ali, S. A. et al. T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128, 1688–1700 (2016).
Young, R. M., Engel, N. W., Uslu, U., Wellhausen, N. & June, C. H. Next-generation CAR T-cell therapies. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-21-1683 (2022).
Krause, A. et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 188, 619–626 (1998).
Ellis, G. I., Sheppard, N. C. & Riley, J. L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 22, 427–447 (2021).
Wellhausen, N., Agarwal, S., Rommel, P. C., Gill, S. I. & June, C. H. Better living through chemistry: CRISPR/Cas engineered T cells for cancer immunotherapy. Curr. Opin. Immunol. 74, 76–84 (2022).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022). Long-term follow-up reveals CAR T cells can persist for 10+ years in patients with cancer.
Fajgenbaum, D. C. & June, C. H. Cytokine storm. N. Engl. J. Med. 383, 2255–2273 (2020).
Flugel, C. L. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62 (2022).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Yeh, J. M. et al. Life expectancy of adult survivors of childhood cancer over 3 decades. JAMA Oncol. 6, 350–357 (2020).
Watanabe, K., Kuramitsu, S., Posey, A. D. & June, C. H. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front. Immunol. 9, 2486 (2018).
Hege, K. M. et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J. Immunother. Cancer 5, 22 (2017). The first trial of CAR T cells in solid tumours.
Zhou, T. et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614 (2020).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018).
Tchou, J. et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 5, 1152–1161 (2017).
Reinhard, K. et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020).
Ma, L. et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019).
Uslu, U. et al. Chimeric antigen receptor T cells as adjuvant therapy for unresectable adenocarcinoma. Sci. Adv. 9, eade2526 (2023).
Good, C. R. et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell 184, 6081–6100 (2021).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015).
Aghajanian, H., Rurik, J. G. & Epstein, J. A. CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat. Metab. 4, 163–169 (2022).
Orlando, E. J. et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 24, 1504–1506 (2018).
Hegde, M. et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 11, 3549 (2020).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. https://doi.org/10.1038/s41591-022-02017-5 (2022). A clinical report of five patients with SLE treated with CD19 CAR T cells.
Baker, D. J. & June, C. H. CAR T therapy extends its reach to autoimmune diseases. Cell 185, 4471–4473 (2022).
Müller, F. et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet 401, 815–818 (2023). A case report of a patient with antisynthetase syndrome treated with CD19 CAR T cells.
Kansal, R. et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 11, eaav1648 (2019).
Jin, X. et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell. Mol. Immunol. 18, 1896–1903 (2020).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Oh, S. et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01637-z (2023).
Parvathaneni, K. & Scott, D. W. Engineered FVIII-expressing cytotoxic T cells target and kill FVIII-specific B cells in vitro and in vivo. Blood Adv. 2, 2332–2340 (2018).
Chen, S. et al. Treatment of allergic eosinophilic asthma through engineered IL-5-anchored chimeric antigen receptor T cells. Cell Discov. 8, 80 (2022).
Zhang, L. et al. Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes. J. Autoimmun. 96, 50–58 (2019).
Beheshti, S. A., Shamsasenjan, K., Ahmadi, M. & Abbasi, B. CAR Treg: a new approach in the treatment of autoimmune diseases. Int. Immunopharmacol. 102, 108409 (2022).
Fritsche, E., Volk, H. D., Reinke, P. & Abou-El-Enein, M. Toward an optimized process for clinical manufacturing of CAR-Treg cell therapy. Trends Biotechnol. 38, 1099–1112 (2020).
Raffin, C., Vo, L. T. & Bluestone, J. A. Treg cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 20, 158–172 (2019).
Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discov. 20, 179–199 (2020).
Sagonowsky, E. The top 20 drugs by worldwide sales in 2020. Fierce Pharma https://www.fiercepharma.com/special-report/top-20-drugs-by-2020-sales (2021).
Henderson, N. C., Rieder, F. & Wynn, T. A. Fibrosis: from mechanisms to medicines. Nature 587, 555–566 (2020).
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019). FAPCAR T cells can treat mouse models of heart failure.
Kakarla, S. et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 21, 1611–1620 (2013).
Wang, L. C. S. et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2, 154–166 (2014).
Lo, A. et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 75, 2800–2810 (2015).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). A single injection of tLNPs generates FAPCAR T cells in vivo and ameliorates heart failure.
Purcell, J. W. et al. LRRC15 is a novel mesenchymal protein and stromal target for antibody–drug conjugates. Cancer Res. 78, 4059–4072 (2018).
Buechler, M. B. et al. Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579 (2021).
van Linthout, S. & Volk, H. D. Immuno-cardio-oncology: killing two birds with one stone? Front. Immunol. 13, 6859 (2022).
Wiley, C. D. & Campisi, J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat. Metab. 3, 1290–1301 (2021).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020). uPAR CAR T cells can treat a variety of senescence-associated conditions.
Prajapati, K., Perez, C., Rojas, L. B. P., Burke, B. & Guevara-Patino, J. A. Functions of NKG2D in CD8+ T cells: an opportunity for immunotherapy. Cell Mol. Immunol. 15, 470–479 (2018).
Sagiv, A. et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 8, 328–344 (2016).
Cerboni, C. et al. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood 110, 606–615 (2007).
Suda, M. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging https://doi.org/10.1038/s43587-021-00151-2 (2021).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Baker, D. J. et al. Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Kim, G. B., Hege, K. & Riley, J. L. CAR talk: how cancer-specific CAR T cells can instruct how to build CAR T cells to cure HIV. Front. Immunol. 10, 2310 (2019).
Hütter, G. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009).
Jensen, B.-E. O. et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29, 583–587 (2023).
Maldini, C. R., Ellis, G. I. & Riley, J. L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 18, 605–616 (2018).
Leibman, R. S. et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog. 13, e1006613 (2017).
Kumaresan, P. R. et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc. Natl Acad. Sci. USA 111, 10660–10665 (2014).
Seif, M. et al. CAR T cells targeting Aspergillus fumigatus are effective at treating invasive pulmonary aspergillosis in preclinical models. Sci. Transl. Med. 14, eabh1209 (2022).
Mo, F. et al. Engineering T-cells to suppress acute GvHD and leukemia relapse after allogeneic hematopoietic stem cell transplantation. Blood https://doi.org/10.1182/BLOOD.2022016052 (2022).
Beier, U. H., Baker, D. J. & Baur, J. A. Thermogenic T cells: a cell therapy for obesity? Am. J. Physiol. Cell Physiol. https://doi.org/10.1152/AJPCELL.00034.2022 (2022).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Lu, Y. et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 26, 732–740 (2020).
Webber, B. R. et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat. Commun. 10, 5222 (2019).
Allen, G. M. et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science 378, eaba1624 (2022).
Yarmarkovich, M. et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021).
Rettko, N. J., Campisi, J. & Wells, J. A. Engineering antibodies targeting p16 MHC-peptide complexes. ACS Chem. Biol. 17, 545–555 (2022).
Qin, V. M. et al. Chimeric antigen receptor beyond CAR-T cells. Cancers 13, 404 (2021).
Pan, K. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 41, 119 (2022).
Tombácz, I. et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 29, 3293–3304 (2021).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020).
Nawaz, W. et al. AAV-mediated in vivo CAR gene therapy for targeting human T-cell leukemia. Blood Cancer J. 11, 119 (2021).
Pfeiffer, A. et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 10, e9158 (2018).
Agarwal, S. et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther. 28, 1783–1794 (2020).
Weidner, T. et al. Genetic in vivo engineering of human T lymphocytes in mouse models. Nat. Protoc. 16, 3210–3240 (2021).
Banskota, S. et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 185, 250–265 (2022).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Themeli, M. et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31, 928–933 (2013).
Benjamin, R. et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 (2020).
Bishop, D. C. et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood 138, 1504–1509 (2021).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Levine, B. L., Miskin, J., Wonnacott, K. & Keir, C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 4, 92–101 (2017).
Ghassemi, S. et al. Enhancing chimeric antigen receptor T cell anti-tumor function through advanced media design. Mol. Ther. Methods Clin. Dev. 18, 595–606 (2020).
Daniels, K. G. et al. Decoding CAR T cell phenotype using combinatorial signaling motif libraries and machine learning. Science 378, 1194–1200 (2022).
Considerations for the development of chimeric antigen receptor (CAR) T cell products. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products (2022).
Kummar, S. et al. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 14, 133–137 (2008).
Acknowledgements
This work was supported by 1P01CA214278 and R01CA226983 (C.H.J.), the Parker Institute for Cancer Immunotherapy and the Centurion Foundation Innovation Fund.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.J.B., Z.A., J.A.B., J.A.E., C.H.J. Visualization: D.J.B., Z.A., J.A.B., J.A.E., C.H.J. Writing: D.J.B., Z.A., J.A.B., J.A.E., C.H.J.
Corresponding authors
Ethics declarations
Competing interests
D.J.B. and Z.A. declare no competing interests. J.A.B. is a consultant to Pfizer and Cytokinetics. J.A.E. is a scientific founder and holds equity in Capstan Therapeutics, which develops therapeutics to reprogram immune cells in vivo. C.H.J. is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives licence revenue from such licences. C.H.J. is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics. C.H.J. is a member of the scientific advisory boards of AC Immune, Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Celldex, Danaher, Decheng, Kite Gilead, Poseida, Verismo, Viracta and WIRB-Copernicus.
Peer review
Peer review information
Nature thanks Kristen Hege and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baker, D.J., Arany, Z., Baur, J.A. et al. CAR T therapy beyond cancer: the evolution of a living drug. Nature 619, 707–715 (2023). https://doi.org/10.1038/s41586-023-06243-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06243-w
This article is cited by
-
The quest for nanoparticle-powered vaccines in cancer immunotherapy
Journal of Nanobiotechnology (2024)
-
GMP-manufactured CRISPR/Cas9 technology as an advantageous tool to support cancer immunotherapy
Journal of Experimental & Clinical Cancer Research (2024)
-
Unleashing CAR T cells to delay metabolic aging
Nature Aging (2024)
-
Natural killer cell therapies
Nature (2024)
-
Cancer Vaccines: A Novel Revolutionized Approach to Cancer Therapy
Indian Journal of Clinical Biochemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.