Abstract
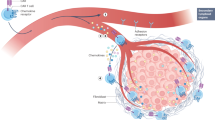
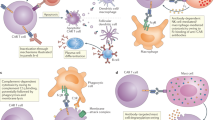
Therapies with genetically modified T cells that express chimeric antigen receptors (CARs) specific for CD19 or B cell maturation antigen (BCMA) are approved to treat certain B cell malignancies. However, translating these successes into treatments for patients with solid tumours presents various challenges, including the risk of clinically serious on-target, off-tumour toxicity (OTOT) owing to CAR T cell-mediated cytotoxicity against non-malignant tissues expressing the target antigen. Indeed, severe OTOT has been observed in various CAR T cell clinical trials involving patients with solid tumours, highlighting the importance of establishing strategies to predict, mitigate and control the onset of this effect. In this Review, we summarize current clinical evidence of OTOT with CAR T cells in the treatment of solid tumours and discuss the utility of preclinical mouse models in predicting clinical OTOT. We then describe novel strategies being developed to improve the specificity of CAR T cells in solid tumours, particularly the role of affinity tuning of target binders, logic circuits and synthetic biology. Furthermore, we highlight control strategies that can be used to mitigate clinical OTOT following cell infusion such as regulating or eliminating CAR T cell activity, exogenous control of CAR expression, and local administration of CAR T cells.
Key points
-
Chimeric antigen receptor (CAR) T cell therapies have led to on-target, off-tumour toxicity (OTOT) in clinical trials involving patients with solid tumours.
-
Preclinical mouse models might provide inaccurate predictions of OTOT in patients, reflecting the need for better models to perform preclinical safety assessments.
-
Logic-gating circuits and synthetic biology approaches to CAR T cell engineering have demonstrated specificity in mice, although many of these approaches remain untested in clinical studies.
-
Methods of controlling CAR T cell activity and responding to unexpected OTOT have the potential to improve safety after infusion.
-
We advocate for robust preclinical analyses of the risks of OTOT and the implementation of control strategies capable of regulating CAR T cell activity in patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
FDA. ABECMA (idecabtagene vicleucel). https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel (2021).
FDA. BREYANZI (lisocabtagene maraleucel). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel (2021).
FDA. KYMRIAH (tisagenlecleucel). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (2021).
FDA. TECARTUS (brexucabtagene autoleucel). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel (2021).
FDA. YESCARTA (axicabtagene ciloleucel). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel (2021).
FDA. CARVYKTI (ciltacabtagene autoleucel). https://www.fda.gov/vaccines-blood-biologics/carvykti (2022).
Amini, L. et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 19, 342–355 (2022).
Logue, J. M. et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica 106, 978–986 (2021).
Uy, N. F. et al. Hypogammaglobulinemia and infection risk in chronic lymphocytic leukemia (CLL) patients treated with CD19-directed chimeric antigen receptor T (CAR-T) cells. Blood 136, 30–32 (2020).
Schubert, M. L. et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 32, 34–48 (2021).
Srivastava, S. et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 35, 489–503.e8 (2019).
Castellarin, M. et al. A rational mouse model to detect on-target, off-tumor CAR T cell toxicity. JCI Insight 5, e136012 (2020).
Smith, J. B. et al. Tumor regression and delayed onset toxicity following B7-H4 CAR T cell therapy. Mol. Ther. 24, 1987–1999 (2016).
Labanieh, L. et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell 185, 1745–1763 (2022).
Lamers, C. H. et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 24, e20–e22 (2006).
Lamers, C. H. J. et al. Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells–a completed study overview. Biochem. Soc. Trans. 44, 951–959 (2016).
Feng, K.-C. et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 10, 4 (2017).
Guo, Y. et al. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin. Cancer Res. 24, 1277–1286 (2018).
Liu, Y. et al. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy 22, 573–580 (2020).
Feng, K. et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 9, 838–847 (2018).
Thistlethwaite, F. C. et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 66, 1425–1436 (2017).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
Zhang, Y. et al. Phase I clinical trial of EGFR-specific CAR-T cells generated by the piggyBac transposon system in advanced relapsed/refractory non-small cell lung cancer patients. J. Cancer Res. Clin. Oncol. 147, 3725–3734 (2021).
Richman, S. A. et al. High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol. Res. 6, 36–46 (2018).
Davenport, A. J. et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl Acad. Sci. USA 115, E2068–E2076 (2018).
Meiraz, A., Garber, O. G., Harari, S., Hassin, D. & Berke, G. Switch from perforin-expressing to perforin-deficient CD8+ T cells accounts for two distinct types of effector cytotoxic T lymphocytes in vivo. Immunology 128, 69–82 (2009).
Hong, L. K. et al. CD30-redirected chimeric antigen receptor T cells target CD30+ and CD30- embryonal carcinoma via antigen-dependent and Fas/FasL interactions. Cancer Immunol. Res. 6, 1274–1287 (2018).
Benmebarek, M.-R. et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. 20, 1283 (2019).
Dufva, O. et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood 135, 597–609 (2020).
Schietinger, A. et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science 314, 304–308 (2006).
Wong, A. J. et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc. Natl Acad. Sci. USA 89, 2965–2969 (1992).
Posey, A. D. Jr et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016).
Heitzeneder, S. et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell 40, 53–69.e9 (2021).
Bosse, K. R. et al. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell 32, 295–309.e12 (2017).
Smith, C. C. et al. Alternative tumour-specific antigens. Nat. Rev. Cancer 19, 465–478 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Li, G. & Wong, A. J. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev. Vaccines 7, 977–985 (2008).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Vitanza, N. A. et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat. Med. 27, 1544–1552 (2021).
Ahmed, N. et al. Autologous HER2 CMV bispecific CAR T cells are safe and demonstrate clinical benefit for glioblastoma in a Phase I trial. J. ImmunoTher. Cancer 3, O11 (2015).
Hegde, M. et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 11, 3549 (2020).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Ivanov, S. et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 158, 905–919 (2001).
Moghimi, B. et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat. Commun. 12, 511 (2021).
Du, H. et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell 35, 221–237.e8 (2019).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
Haas, A. R. et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 27, 1919–1929 (2019).
Maus, M. V. et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 1, 26–31 (2013).
Beatty, G. L. et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2, 112–120 (2014).
Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
Straathof, K. et al. Antitumor activity without on-target off-tumor toxicity of GD2–chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med. 12, eabd6169 (2020).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Gargett, T. et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol. Ther. 24, 1135–1149 (2016).
Lamers, C. H. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Ahmed, N. et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015).
Ahmed, N. et al. HER2-specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 3, 1094–1101 (2017).
Brown, C. E. et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 21, 4062–4072 (2015).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e17 (2020).
Van Oekelen, O. et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 27, 2099–2103 (2021).
Mount, C. W. et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med. 24, 572–579 (2018).
Park, S. et al. Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Sci. Rep. 7, 14366 (2017).
Kosti, P. et al. Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors. Cell Rep. Med. 2, 100227 (2021).
Majzner, R. G., Weber, E. W., Lynn, R. C., Xu, P. & Mackall, C. L. Neurotoxicity associated with a high-affinity GD2 CAR-letter. Cancer Immunol. Res. 6, 494–495 (2018).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Kato, D. et al. GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab. eLlife 9, e49392 (2020).
Fedorov, V. D., Themeli, M. & Sadelain, M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 5, 215ra172 (2013).
Caruso, H. G. et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 75, 3505–3518 (2015).
Hernandez-Lopez, R. A. et al. T cell circuits that sense antigen density with an ultrasensitive threshold. Science 371, 1166–1171 (2021).
Cui, X. et al. Dissecting the immunosuppressive tumor microenvironments in Glioblastoma-on-a-Chip for optimized PD-1 immunotherapy. eLlife 9, e52253 (2020).
Ingber, D. E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23, 467–491 (2022).
Liu, X. et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 75, 3596–3607 (2015).
Watanabe, K. et al. Excessively high-affinity single-chain fragment variable region in a chimeric antigen receptor can counteract T-cell proliferation. Blood 124, 4799 (2014).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Hegde, M. et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 126, 3036–3052 (2016).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Alabanza, L. et al. Function of Novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol. Ther. 25, 2452–2465 (2017).
Majzner, R. G. et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 10, 702–723 (2020).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Wilkie, S. et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 32, 1059–1070 (2012).
Kloss, C. C., Condomines, M., Cartellieri, M., Bachmann, M. & Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 31, 71–75 (2013).
Zhang, E. et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. J. Hematol. Oncol. 11, 102 (2018).
Sukumaran, S. et al. Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Discov. 8, 972–987 (2018).
Zhang, W. et al. Abstract PO074: Logic-gating HER2 CAR-T to the tumor microenvironment mitigates on-target, off-tumor toxicity without compromising cytotoxicity against HER2-over-expressing tumors. Cancer Immunol. Res. 9, PO074 (2021).
Vaupel, P. & Multhoff, G. Revisiting the Warburg effect: historical dogma versus current understanding. J. Physiol. 599, 1745–1757 (2021).
Juillerat, A. et al. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 7, 39833 (2017).
Han, X. et al. Masked chimeric antigen receptor for tumor-specific activation. Mol. Ther. 25, 274–284 (2017).
Liu, C., Sun, C., Huang, H., Janda, K. & Edgington, T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 63, 2957–2964 (2003).
Liu, G., Shuman, M. A. & Cohen, R. L. Co-expression of urokinase, urokinase receptor and PAI-1 is necessary for optimum invasiveness of cultured lung cancer cells. Int. J. Cancer 60, 501–506 (1995).
Singhal, R. & Shah, Y. M. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505 (2020).
Zhang, J. L. et al. Measurement of renal tissue oxygenation with blood oxygen level-dependent MRI and oxygen transit modeling. Am. J. Physiol. Ren. Physiol. 306, F579–F587 (2014).
Dumas, S. J. et al. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat. Rev. Nephrol. 17, 441–464 (2021).
Roybal, K. T. et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167, 419–432.e416 (2016).
Roybal, K. T. et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–779 (2016).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 164, 780–791 (2016).
Hyrenius-Wittsten, A. et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci. Transl. Med. 13, eabd8836 (2021).
Choe, J. H. et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci. Transl. Med. 13, eabe7378 (2021).
Williams, J. Z. et al. Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science 370, 1099–1104 (2020).
Wagner, D. L. et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 18, 379–393 (2021).
Zhu, I. et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell 185, 1431–1443 (2022).
Feldmann, A. et al. Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR”. Oncotarget 8, 31368–31385 (2017).
Albert, S. et al. A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. Oncoimmunology 6, e1287246 (2017).
Mitwasi, N. et al. Development of novel target modules for retargeting of UniCAR T cells to GD2 positive tumor cells. Oncotarget 8, 108584–108603 (2017).
Jureczek, J. et al. Highly efficient targeting of EGFR-expressing tumor cells with UniCAR T cells via target modules based on cetuximab(®). OncoTargets Ther. 13, 5515–5527 (2020).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438.e11 (2018).
Salzer, B. et al. Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function. Nat. Commun. 11, 4166 (2020).
Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 69, 4–10 (2005).
Richards, R. M. et al. NOT-Gated CD93 CAR T cells effectively target AML with minimized endothelial cross-reactivity. Blood Cancer Discov. 2, 648–665 (2021).
Sandberg, M. L. et al. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci. Transl. Med. 14, eabm0306 (2022).
McGranahan, N. et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171, 1259–1271.e11 (2017).
De Mattos-Arruda, L. et al. The genomic and immune landscapes of lethal metastatic breast cancer. Cell Rep. 27, 2690–2708.e10 (2019).
Dong, L.-Q. et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 72, 896–908 (2020).
Hamburger, A. E. et al. Engineered T cells directed at tumors with defined allelic loss. Mol. Immunol. 128, 298–310 (2020).
Tokatlian, T. et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J. Immunother. Cancer 10, e003826 (2022).
Hwang, M. S. et al. Targeting loss of heterozygosity for cancer-specific immunotherapy. Proc. Natl Acad. Sci. USA 118, e2022410118 (2021).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Thompson, J. A. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J. Natl Compr. Canc. Netw. 16, 594–596 (2018).
Weber, E. W. et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 3, 711–717 (2019).
Mestermann, K. et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 11, eaau5907 (2019).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021).
Porkka, K. et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 112, 1005–1012 (2008).
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Stavrou, M. et al. A rapamycin-activated Caspase 9-based suicide gene. Mol. Ther. 26, 1266–1276 (2018).
Duong, M. T. et al. Two-dimensional regulation of CAR-T cell therapy with orthogonal switches. Mol. Ther. Oncolytics 12, 124–137 (2019).
Philip, B. et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124, 1277–1287 (2014).
Wang, X. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
de Witte, M. A. et al. An inducible caspase 9 safety switch can halt cell therapy-induced autoimmune disease. J. Immunol. 180, 6365–6373 (2008).
Foster, M. C. et al. Utility of a safety switch to abrogate CD19.CAR T-cell-associated neurotoxicity. Blood 137, 3306–3309 (2021).
Paszkiewicz, P. J. et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest. 126, 4262–4272 (2016).
Villaseñor, R. et al. Trafficking of endogenous immunoglobulins by endothelial cells at the blood-brain barrier. Sci. Rep. 6, 25658 (2016).
Wehler, T. C. et al. Cetuximab-induced skin exanthema: prophylactic and reactive skin therapy are equally effective. J. Cancer Res. Clin. Oncol. 139, 1667–1672 (2013).
Koristka, S. et al. Anti-CAR-engineered T cells for epitope-based elimination of autologous CAR T cells. Cancer Immunol. Immunother. 68, 1401–1415 (2019).
Juillerat, A. et al. Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC Biotechnol. 19, 44–44 (2019).
Sahillioglu, A. C., Toebes, M., Apriamashvili, G., Gomez, R. & Schumacher, T. N. CRASH-IT switch enables reversible and dose-dependent control of TCR and CAR T-cell function. Cancer Immunol. Res. 9, 999–1007 (2021).
Li, H. S. et al. High-performance multiplex drug-gated CAR circuits. Cancer Cell https://doi.org/10.1016/j.ccell.2022.08.008 (2022).
Jan, M. et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci. Transl. Med. 13, eabb6295 (2021).
Wu, C. Y., Roybal, K. T., Puchner, E. M., Onuffer, J. & Lim, W. A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350, aab4077 (2015).
Lohmueller, J. et al. Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting. Preprint at https://doi.org/10.1101/2020.01.17.909895 (2020).
Mata, M. et al. Inducible activation of MyD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 7, 1306–1319 (2017).
Kotter, B. et al. Titratable pharmacological regulation of CAR T cells using zinc finger-based transcription factors. Cancers 13, 4741 (2021).
Hotblack, A. et al. Tunable control of CAR T cell activity through tetracycline mediated disruption of protein-protein interaction. Sci. Rep. 11, 21902 (2021).
Richman, S. A. et al. Ligand-induced degradation of a CAR permits reversible remote control of CAR T cell activity in vitro and in vivo. Mol. Ther. 28, 1600–1613 (2020).
Zajc, C. U. et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl Acad. Sci. USA 117, 14926 (2020).
Leung, W.-H. et al. Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization. JCI Insight 5, e124430 (2019).
Monteys, A. M. et al. Regulated control of gene therapies by drug-induced splicing. Nature 596, 291–295 (2021).
Pan, Y. et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc. Natl Acad. Sci. USA 115, 992–997 (2018).
Wu, Y. et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 5, 1336–1347 (2021).
Huang, Z. et al. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci. Adv. 6, eaay9209 (2020).
Kobayashi, A. et al. Light-controllable binary switch activation of CAR T cells. ChemMedChem 17, e202100722 (2022).
Priceman, S. J. et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin. Cancer Res. 24, 95–105 (2018).
Tchou, J. et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 5, 1152–1161 (2017).
Hiltbrunner, S. et al. Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: first report of FAPME, a phase I clinical trial. Ann. Oncol. 32, 120–121 (2021).
Donovan, L. K. et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 26, 720–731 (2020).
Nellan, A. et al. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J. Immunother. Cancer 6, 30 (2018).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
Theruvath, J. et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 26, 712–719 (2020).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021).
Haydar, D. et al. Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. NeuroOncology 23, 999–1011 (2021).
Srivastava, S. & Riddell, S. R. Chimeric antigen receptor T cell therapy: challenges to bench-to-bedside efficacy. J. Immunol. 200, 459–468 (2018).
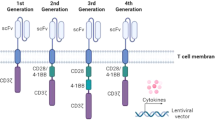
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 4, 676-84 (2004).
Xiao, Q. et al. Size-dependent activation of CAR-T cells. Sci. Immunol. 7, eabl3995 (2022).
Alizadeh, D. et al. IFNγ is critical for CAR T cell-mediated myeloid activation and induction of endogenous immunity. Cancer Discov. 11, 2248–2265 (2021).
Chmielewski, M., Kopecky, C., Hombach, A. A. & Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71, 5697–5706 (2011).
Zhang, L. et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol. Ther. 19, 751–759 (2011).
Uhlen, M. et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28, 1248–1250 (2010).
Nagata, Y. et al. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 267, 12082–12089 (1992).
Acknowledgements
The authors thank K. T. Roybal (University of California, San Francisco, USA) for his helpful comments on an earlier draft of the manuscript. They thank A. Cadinanos-Garai (University of Southern California, USA) for her critical review of the manuscript and figures. The work of G.K. is supported by R01NS121249 from NINDS and the Assisi Foundation of Memphis. The work of M.A. is supported, in part, by award P30CA014089 from the US NIH National Cancer Institute (NCI).
Author information
Authors and Affiliations
Contributions
C.L.F, D.L.W. and M.A. researched data for the manuscript, all authors made a substantial contribution to discussions of content. C.L.F., D.L.W. and M.A. wrote the manuscript, and all authors reviewed and/or edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
R.G.M. has acted as an adviser and/or consultant of Aptorum Group, Arovella Therapeutics, Immunai, Innervate Radiopharmaceuticals, Link Cell Therapies, Lyell Immunopharma, NKarta, Syncopation Life Sciences, and Zai lab and is a co-founder of and holds equity in Syncopation Life Sciences and Link Cell Therapies. G.K. has patent applications in the field of immunotherapy. G.D. has acted as a scientific adviser and/or consultant of Bellicum Pharmaceutical and Catamaran and Tessa Therapeutics and holds patents in the field of CAR T cells. S.R.R. has acted as a scientific adviser of Adaptive Biotechnologies and Juno Therapeutics, is a co-founder of and has intellectual property licensed to Lyell Immunopharma and Juno Therapeutics, and holds shares in and has received research funding from Lyell Immunopharma. C.L.F., D.L.W. and M.A. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks L. Shen, M. Maus, and the other, anonymous, reviewer(s), for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flugel, C.L., Majzner, R.G., Krenciute, G. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol 20, 49–62 (2023). https://doi.org/10.1038/s41571-022-00704-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-022-00704-3
This article is cited by
-
Functional CRISPR screens in T cells reveal new opportunities for cancer immunotherapies
Molecular Cancer (2024)
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy
Cellular & Molecular Immunology (2024)
-
Biomaterials to enhance adoptive cell therapy
Nature Reviews Bioengineering (2024)
-
In vivo manufacture and manipulation of CAR-T cells for better druggability
Cancer and Metastasis Reviews (2024)