Abstract
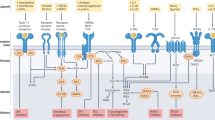
Tyrosine kinase 2 (TYK2) is a member of the JAK kinase family of intracellular signalling molecules. By participating in signalling pathways downstream of type I interferons, IL-12, IL-23 and IL-10, TYK2 elicits a distinct set of immune events to JAK1, JAK2 and JAK3. TYK2 polymorphisms have been associated with susceptibility to various rheumatic diseases including systemic lupus erythematosus and dermatomyositis. In vitro and animal studies substantiate these findings, highlighting a role for TYK2 in diseases currently managed by antagonists of cytokines that signal through TYK2. Various inhibitors of TYK2 have now been studied in human disease, and one of these inhibitors, deucravacitinib, has now been approved for the treatment of psoriasis. Phase II trials of deucravacitinib have also reported positive results in the treatment of psoriatic arthritis and systemic lupus erythematosus, with a preliminary safety profile that seems to differ from that of the JAK1, JAK2 and JAK3 inhibitors. Two other inhibitors of TYK2, brepocitinib and ropsacitinib, are also in earlier stages of clinical trials. Overall, TYK2 inhibitors hold promise for the treatment of a distinct spectrum of autoimmune diseases and could potentially have a safety profile that differs from other JAK inhibitors.
Key points
-
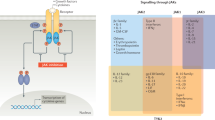
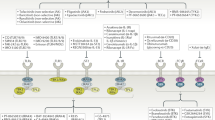
Tyrosine kinase 2 (TYK2) is a member of the Janus kinase (JAK) family and is involved in signalling downstream of a distinct set of cytokines, including IL-12, IL-23, type I interferons and IL-10.
-
TYK2 has a potential role in diseases in which these cytokines are pathogenic; given its limited cytokine profile, inhibitors of TYK2 could have a different safety profile from other JAK inhibitors.
-
The selective TYK2 inhibitor deucravacitinib has a unique mode of action that involves allosteric inhibition of TYK2 via binding to a pseudokinase domain.
-
Other known inhibitors of TYK2, including brepocitinib and ropsacitinib, bind to the kinase domain of TYK2, and have inhibitory activity against other JAK kinases.
-
Phase III clinical trials of deucravacitinib have resulted in its approval for the treatment of plaque psoriasis, and phase II trials in psoriatic arthritis and systemic lupus erythematosus were positive.
-
Preliminary safety data to date suggest that deucravacitinib has a distinct safety profile compared with other JAK inhibitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Conn, D. L. The story behind the use of glucocorticoids in the treatment of rheumatoid arthritis. Semin. Arthritis Rheum. 51, 15–19 (2021).
Cain, D. W. & Cidlowski, J. A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247 (2017).
McLornan, D. P., Pope, J. E., Gotlib, J. & Harrison, C. N. Current and future status of JAK inhibitors. Lancet 398, 803–816 (2021).
O’Shea, J. J. & Gadina, M. Selective Janus kinase inhibitors come of age. Nat. Rev. Rheumatol. 15, 74–75 (2019).
Liu, C., Kieltyka, J., Fleischmann, R., Gadina, M. & O’Shea, J. J. A decade of JAK inhibitors: what have we learned and what may be the future? Arthritis Rheumatol. 73, 2166–2178 (2021).
Shuai, K. & Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 (2003).
Strobl, B., Stoiber, D., Sexl, V. & Mueller, M. Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity. Front. Biosci. 16, 3214–3232 (2011).
Burke, J. R. et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci. Transl. Med. 11 (2019).
Fuchs, S. et al. Tyrosine kinase 2 is not limiting human antiviral type III interferon responses. Eur. J. Immunol. 46, 2639–2649 (2016).
Teng, M. W. et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 21, 719–729 (2015).
Yang, K., Oak, A. S. W. & Elewski, B. E. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am. J. Clin. Dermatol. 22, 173–192 (2021).
Singh, J. A. et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 71, 5–32 (2019).
Gossec, L. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 79, 700–712 (2020).
Menter, A. et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 80, 1029–1072 (2019).
Sarabia, S., Ranjith, B., Koppikar, S. & Wijeratne, D. T. Efficacy and safety of JAK inhibitors in the treatment of psoriasis and psoriatic arthritis: a systematic review and meta-analysis. BMC Rheumatol. 6, 71 (2022).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Furie, R. A. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 1, e208–e219 (2019).
Kalunian, K. C. et al. A randomized, placebo-controlled phase III extension trial of the long-term safety and tolerability of anifrolumab in active systemic lupus erythematosus. Arthritis Rheumatol. 75, 253–265 (2023).
Hagberg, N. et al. The STAT4 SLE risk allele rs7574865[T] is associated with increased IL-12-induced IFN-γ production in T cells from patients with SLE. Ann. Rheum. Dis. 77, 1070–1077 (2018).
Parkes, M., Cortes, A., van Heel, D. A. & Brown, M. A. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat. Rev. Genet. 14, 661–673 (2013).
Sigurdsson, S. et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am. J. Hum. Genet. 76, 528–537 (2005).
Cunninghame Graham, D. S. et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 7, e1002341 (2011).
Yin, Q. et al. Comprehensive assessment of the association between genes on JAK-STAT pathway (IFIH1, TYK2, IL-10) and systemic lupus erythematosus: a meta-analysis. Arch. Dermatol. Res. 310, 711–728 (2018).
Diogo, D. et al. TYK2 protein-coding variants protect against rheumatoid arthritis and autoimmunity, with no evidence of major pleiotropic effects on non-autoimmune complex traits. PLoS One 10, e0122271 (2015).
Lopez-Isac, E. et al. Influence of TYK2 in systemic sclerosis susceptibility: a new locus in the IL-12 pathway. Ann. Rheum. Dis. 75, 1521–1526 (2016).
Bossini-Castillo, L. et al. A GWAS follow-up study reveals the association of the IL12RB2 gene with systemic sclerosis in Caucasian populations. Hum. Mol. Genet. 21, 926–933 (2012).
Hu, L. et al. Interleukin-22 from type 3 innate lymphoid cells aggravates lupus nephritis by promoting macrophage infiltration in lupus-prone mice. Front. Immunol. 12, 584414 (2021).
Yang, X. et al. Increased interleukin-22 levels in lupus nephritis and its associated with disease severity: a study in both patients and lupus-like mice model. Clin. Exp. Rheumatol. 37, 400–407 (2018).
Jani, M. et al. Genotyping of immune-related genetic variants identifies TYK2 as a novel associated locus for idiopathic inflammatory myopathies. Ann. Rheum. Dis. 73, 1750–1752 (2014).
Hromadova, D., Elewaut, D., Inman, R. D., Strobl, B. & Gracey, E. From science to success? targeting tyrosine kinase 2 in spondyloarthritis and related chronic inflammatory diseases. Front. Genet. 12, 685280 (2021).
Takeda. Takeda announces positive results in phase 2b study of investigational TAK-279, an oral, once-daily TYK2 inhibitor, in people with moderate-to-severe plaque psoriasis [press release]. Takeda.com https://www.takeda.com/newsroom/newsreleases/2023/takeda-announces-positive-results-in-phase-2b-study-of-investigational-tak-279/ (2023).
Papp, K. et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N. Engl. J. Med. 379, 1313–1321 (2018).
Armstrong, A. W. et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 88, 29–39 (2023).
Strober, B. et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 88, 40–51 (2023).
Mease, P. J. et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 81, 815–822 (2022).
Felson, D. T. et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheumatol. 38, 727–735 (1995).
Furie, R. A. et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheumatol. 61, 1143–1151 (2009).
Morand, E. et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 75, 242–252 (2023).
Connelly, K., Golder, V., Kandane-Rathnayake, R. & Morand, E. F. Clinician-reported outcome measures in lupus trials: a problem worth solving. Lancet Rheumatol. 3, e595–e603 (2021).
Wu, C. et al. Pharmacodynamic changes in SLE relevant gene expression induced by deucravacitinib in patients enrolled in the phase 2. Arthritis Rheumatol. 75 https://doi.org/10.1002/art.42700 (2023).
Wallace, D. J. et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann. Rheum. Dis. 73, 183–190 (2014).
Klein, R. et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch. Dermatol. 147, 203–208 (2011).
Golder, V. et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatol. 1, e95–e102 (2019).
Petri, M. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet 401, 1011–1019 (2023).
Morand, E. F. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet 401, 1001–1010 (2023).
Dorner, T. et al. Baricitinib-associated changes in global gene expression during a 24-week phase II clinical systemic lupus erythematosus trial implicates a mechanism of action through multiple immune-related pathways. Lupus Sci. Med. 7, e000424 (2020).
Vogel, A. et al. JAK1 signaling in dendritic cells promotes peripheral tolerance in autoimmunity through PD-L1-mediated regulatory T cell induction. Cell Rep. 38, 110420 (2022).
Morand, E. F., Fernandez-Ruiz, R., Blazer, A. & Niewold, T. B. Advances in the management of systemic lupus erythematosus. BMJ 383, e073980 (2023).
ClinicalTrials.gov, United States National Library of Medicine. Efficacy and Safety of Deucravacitinib Versus Placebo in Participants With Moderate-to-severe Scalp Psoriasis, https://clinicaltrials.gov/study/NCT05478499 (2023).
ClinicalTrials.gov. United States National Library of Medicine. A Study to Assess Effectiveness and Safety of Deucravacitinib Compared With Placebo in Participants With Active Systemic Lupus Erythematosus (SLE) (POETYK SLE-1), https://clinicaltrials.gov/study/NCT05617677 (2024).
ClinicalTrials.gov. United States National Library of Medicine. A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs, https://clinicaltrials.gov/study/NCT04908202 (2024).
ClinicalTrials.gov, United States National Library of Medicine. A Study to Evaluate Efficacy and Safety of Deucravacitinib in Participants With Active Discoid and/ or Subacute Cutaneous Lupus Erythematosus (DLE/SCLE), https://clinicaltrials.gov/study/NCT04857034 (2024).
ClinicalTrials.gov, United States National Library of Medicine. A Study to Evaluate Efficacy and Safety of Deucravacitinib in Participants With Alopecia Areata https://clinicaltrials.gov/study/NCT05556265 (2024).
ClinicalTrials.gov, United States National Library of Medicine. A Study to Evaluate the Long-term Safety and Efficacy of Deucravacitinib in Participants With Crohn’s Disease or Ulcerative Colitis. https://clinicaltrials.gov/study/NCT04877990 (2023).
ClinicalTrials.gov, United States National Library of Medicine. A Study of the Safety, Efficacy, and Biomarker Response of BMS-986165 in Participants With Moderate to Severe Ulcerative Colitis. https://clinicaltrials.gov/study/NCT04613518 (2023).
Danese, S. et al. DOP42 efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in patients with moderately-to-severely active ulcerative colitis: 12-week results from the Phase 2 LATTICE-UC study. J. Crohn’s Colitis 16, i091–i092 (2022).
Banfield, C. et al. The safety, tolerability, pharmacokinetics, and pharmacodynamics of a TYK2/JAK1 inhibitor (PF-06700841) in healthy subjects and patients with plaque psoriasis. J. Clin. Pharmacol. 58, 434–447 (2018).
Singh, R. S. P. et al. Safety and pharmacokinetics of the oral TYK2 inhibitor PF-06826647: a phase I, randomized, double-blind, placebo-controlled, dose-escalation study. Clin. Transl. Sci. 14, 671–682 (2021).
Gerstenberger, B. S. et al. Discovery of tyrosine kinase 2 (TYK2) inhibitor (PF-06826647) for the treatment of autoimmune diseases. J. Med. Chem. 63, 13561–13577 (2020).
Tehlirian, C. et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 87, 333–342 (2022).
Forman, S. B. et al. TYK2/JAK1 inhibitor PF-06700841 in patients with plaque psoriasis: phase IIa, randomized, double-blind, placebo-controlled trial. J. Invest. Dermatol. 140, 2359–2370 e2355 (2020).
Mease, P. et al. Efficacy and safety of the TYK2/JAK1 inhibitor brepocitinib for active psoriatic arthritis: a phase IIb randomized controlled trial. Arthritis Rheumatol. 75, 1370–1380 (2023).
Sandborn, W. J. et al. Oral ritlecitinib and brepocitinib for moderate-to-severe ulcerative colitis: results from a randomized, phase 2b study. Clin. Gastroenterol. Hepatol. 21, 2616–2628.e7 (2023).
Minegishi, Y. et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25, 745–755 (2006).
Ojuawo, O., Allen, R., Hagan, G. & Piracha, S. Disseminated tuberculosis associated with deficient interleukin-23/tyrosine kinase 2 signalling. BMJ Case Rep. 15, e250479 (2022).
Dendrou, C. A. et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci. Transl. Med. 8, 363ra149 (2016).
Wang, Y. et al. COVID-19 and systemic lupus erythematosus genetics: a balance between autoimmune disease risk and protection against infection. PLoS Genet. 18, e1010253 (2022).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020).
Nogueira, M., Puig, L. & Torres, T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs 80, 341–352 (2020).
Fleischmann, R. et al. Safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor: as assessed by laboratory parameters — results from a phase 2 trial in psoriatic arthritis and 2 phase 3 trials in psoriasis. Arthritis Rheumatol. 74 https://doi.org/10.1002/art.42355 (2022).
Catlett, I. M. et al. First-in-human study of deucravacitinib: a selective, potent, allosteric small-molecule inhibitor of tyrosine kinase 2. Clin. Transl. Sci. 16, 151–164 (2023).
Hak, A. E., Karlson, E. W., Feskanich, D., Stampfer, M. J. & Costenbader, K. H. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheumatol. 61, 1396–1402 (2009).
Tummala, R. et al. Safety profile of anifrolumab in patients with active SLE: an integrated analysis of phase II and III trials. Lupus Sci. Med. 8, e000464 (2021).
Fleischmann, R. et al. Safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor: as assessed by laboratory parameters — results from a phase 2 trial in psoriatic arthritis and 2 phase 3 trials in psoriasis. Arthritis Rheumatol. 74 (2022).
Tabata, M. M. et al. The type I interferon signature reflects multiple phenotypic and activity measures in dermatomyositis. Arthritis Rheumatol. 75, 1842–1849 (2023).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.F. declares that he has acted as a consultant for AbbVie, Amgen, Atomwise, BMS, Galvani, Galapagos, Gilead, GSK, Immunovant, Janssen, Eli Lilly. Novartis, Pfizer, UCB and Vyne; serves on the data safety monitoring boards for Celltrion, EMDSerano and Kiniksa; and has received clinical trial grants from AbbVie, Arthrosi, Biogen, BMS, Eli Lilly, Flexion, Galvani, Genentech, Gilead, GSK, Horizon, Janssen, Novartis, Oletec, Priovant, Scipher, Selecta, UCB and Viela. Y.T. declares that he has received speaking fees and/or honoraria from AbbVie, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers, Chugai, Daiichi-Sankyo, Eli Lilly, Eisai, Gilead, GlaxoSmithKline, Mitsubishi-Tanabe and Pfizer; and research grants from Asahi-Kasei, AbbVie, Behringer-Ingelheim, Chugai, Daiichi-Sankyo, Eisai and Takeda. D.G. declares that she has received grant support from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer and UCB; and consulting fees from AbbVie, Amgen, BMS, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB. E.M. declares that he has received research grants from AbbVie, Amgen, AstraZeneca, Biogen, Bristol Myers Squibb, Eli Lilly, EMD Serono, Genentech, GSK, Janssen, Takeda and UCB; and advisory and/or honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, EMD Serono, Genentech, GSK, Gilead, Novartis, Takeda and Zenas. J.F.M. declares that he has acted as a consultant for AbbVie, Biogen, Celgene, Dermavant, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer and UCB.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Peter Nash and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morand, E., Merola, J.F., Tanaka, Y. et al. TYK2: an emerging therapeutic target in rheumatic disease. Nat Rev Rheumatol 20, 232–240 (2024). https://doi.org/10.1038/s41584-024-01093-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01093-w