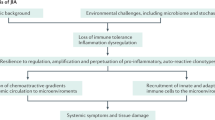
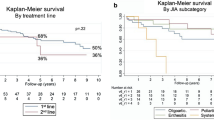
Abstract
In the past two decades, the treatment of juvenile idiopathic arthritis (JIA) has evolved markedly, owing to the availability of a growing number of novel, potent and relatively safe therapeutic agents and the shift of management strategies towards early achievement of disease remission. However, JIA encompasses a heterogeneous group of diseases that require distinct treatment approaches. Furthermore, some old drugs, such as methotrexate, sulfasalazine and intraarticular glucocorticoids, still maintain an important therapeutic role. In the past 5 years, information on the efficacy and safety of drug therapies for JIA has been further enriched through the accomplishment of several randomized controlled trials of newer biologic and synthetic targeted DMARDs. In addition, a more rational therapeutic approach has been fostered by the promulgation of therapeutic recommendations and guidelines. A multinational collaborative effort has led to the development of the recommendations for the treat-to-target strategy in JIA. There is currently increasing interest in establishing the optimal time and modality for discontinuation of treatment in children with JIA who achieve sustained clinical remission. The aim of this Review is to summarize the current evidence and discuss the therapeutic approaches to the management of non-systemic phenotypes of JIA, including oligoarthritis, polyarthritis, enthesitis-related arthritis and psoriatic arthritis.
Key points
-
In the past two decades, important progress has been made in the management of juvenile idiopathic arthritis, including the availability of new therapeutic agents and the shift towards early aggressive interventions.
-
Several randomized controlled trials, therapeutic recommendations and consensus treatment plans have facilitated a more rational approach to therapy.
-
Contemporary therapeutic goals include early achievement of disease control, sparing use of glucocorticoids and the prevention of disease-related and treatment-related morbidity.
-
The application of the treat-to-target strategy, an innovative treatment modality that has already been explored successfully in pivotal therapeutic studies, is now garnering increased interest.
-
The variability in clinical presentation and course of juvenile idiopathic arthritis implies that the therapeutic choices, optimal targets and treatment strategy might be different across disease categories.
-
The research agenda calls for innovative trials that improve remission rates and pave the way for refined precision studies, personalized medicine and, ultimately, future prevention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Levinson, J. E. & Wallace, C. A. Dismantling the pyramid. J. Rheumatol. 33, 6–10 (1992).
Tambralli, A. et al. High doses of infliximab in the management of juvenile idiopathic arthritis. J. Rheumatol. 40, 1749–55 (2013).
Ravelli, A. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann. Rheum. Dis. 77, 819–828 (2018).
Giancane, G. et al. Disease activity and damage in juvenile idiopathic arthritis: methotrexate era versus biologic era. Arthritis Res. Ther. 21, 168 (2019).
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Martini, A. It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann. Rheum. Dis. 71, 1437–1439 (2012).
Martini, A. et al. Juvenile idiopathic arthritis. Nat. Rev. Dis. Prim. 8, 5 (2022).
Martini, A. Toward new classification criteria for juvenile idiopathic arthritis: first steps, Pediatric Rheumatology International Trials Organization international consensus. J. Rheumatol. 46, 190–197 (2019).
Beukelman, T. & Nigrovic, P. Juvenile idiopathic arthritis: an idea whose time has gone? J. Rheumatol. 46, 124–126 (2019).
Nigrovic, P. A. et al. Biological classification of childhood arthritis: roadmap to a molecular nomenclature. Nat. Rev. Rheumatol. 17, 257–269 (2021).
Hinze, C. H., Foell, D. & Kessel, C. Treatment of systemic juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 19, 778–789 (2023).
Ravelli, A. et al. Antinuclear antibody-positive patients should be grouped as a separate category in the classification of juvenile idiopathic arthritis. Arthritis Rheum. 63, 267–275 (2011).
Ringold, S. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res. 71, 717–734 (2019).
Onel, K. B. et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 74, 553–569 (2022).
Scott, C. et al. A reappraisal of intra-articular corticosteroid therapy in juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 28, 774–781 (2010).
Klein, A. et al. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry. Arthritis Care Res. 64, 1349–1356 (2012).
Raab, A. et al. Outcome of children with oligoarticular juvenile idiopathic arthritis compared to polyarthritis on methotrexate- data of the German BIKER registry. Pediatr. Rheumatol. Online J. 19, 41 (2021).
Bakry, R., Klein, M. A. & Horneff, G. Oral or parenteral methotrexate for the treatment of polyarticular juvenile idiopathic arthritis. Eur. J. Rheumatol. 9, 197–205 (2022).
Dupuis, L. L., Koren, G., Silverman, E. D. & Laxer, R. M. Influence of food on the bioavailability of oral methotrexate in children. J. Rheumatol. 22, 1570–1573 (1995).
Jundt, J. W., Browne, B. A., Fiocco, G. P., Steele, A. D. & Mock, D. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J. Rheumatol. 20, 1845–1849 (1993).
Hissink Muller, P. et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann. Rheum. Dis. 78, 51–59 (2019).
Hinze, C., Gohar, F. & Foell, D. et al. Management of juvenile idiopathic arthritis: hitting the target. Nat. Rev. Rheumatol. 11, 290–300 (2015).
Ter Haar, N. M. et al. Treatment to target using recombinant interleukin-1 receptor antagonist as first-line monotherapy in new-onset systemic juvenile Idiopathic arthritis: results from a five-year follow-up study. Arthritis Rheumatol. 71, 1163–1173 (2019).
Klein, A. et al. Treat-to-target study for improved outcome in polyarticular juvenile idiopathic arthritis. Ann. Rheum. Dis. 79, 969–974 (2020).
Rosina, S., Rebollo-Giménez, A. I., Consolaro, A. & Ravelli, A. Treat-to-target in pediatric rheumatic diseases. Curr. Rheumatol. Rep. 25, 226–235 (2023).
Wallace, C., Giannini, E., Huang, B., Itert, L. & Ruperto, N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. 63, 929–936 (2011).
Magni-Manzoni, S. et al. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum. 59, 1120–1127 (2008).
Consolaro, A. et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum. 64, 2366–2374 (2012).
Trincianti, C. et al. Definition and validation of the American College of Rheumatology 2021 Juvenile Arthritis Disease Activity Score Cutoffs for disease activity states in juvenile idiopathic arthritis. Arthritis Rheumatol. 73, 1966–1975 (2021).
Sherry, D. D., Stein, L. D., Reed, A. M., Schanberg, L. E. & Kredich, D. W. Prevention of leg length discrepancy in young children with pauciarticular juvenile rheumatoid arthritis by treatment with intraarticular steroids. Arthritis Rheum. 42, 2330–2334 (1999).
Ravelli, A. et al. Intra-articular corticosteroids versus intra-articular corticosteroids plus methotrexate in oligoarticular juvenile idiopathic arthritis: a multicentre, prospective, randomised, open-label trial. Lancet 389, 909–916 (2017).
Stoustrup, P. et al. Management of orofacial manifestations of juvenile idiopathic arthritis: interdisciplinary consensus-based recommendations. Arthritis Rheumatol. 75, 4–14 (2023).
Horneff, G. et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann. Rheum. Dis. 73, 1114–1122 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03841357 (2024).
O’Dell, J. R. et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N. Engl. J. Med. 334, 1287–1291 (1996).
Tynjälä, P. et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann. Rheum. Dis. 70, 1605–1612 (2011).
Alexeeva, E. I. et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin. Rheumatol. 30, 1163–1172 (2011).
Kearsley-Fleet, L. et al. Use and effectiveness of rituximab in children and young people with juvenile idiopathic arthritis in a cohort study in the United Kingdom. Rheumatology 58, 331–335 (2019).
Marino, A., Orsini, F., Pregnolato, F. & Cimaz, R. Tumor necrosis factor-α inhibition before and after rituximab treatment in juvenile idiopathic arthritis: what shall we expect? A pilot study. J. Rheumatol. 49, 654–656 (2022).
De Benedetti, F. et al. Sarilumab, a human monoclonal antibody to the interleukin-6 receptor, in polyarticular-course juvenile idiopathic arthritis: a 12-week, multinational, open-label, dose-finding study. Arthritis Rheumatol. 71 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02776735 (2023).
Amarilyo, G. et al. Biological agents in polyarticular juvenile idiopathic arthritis: a meta-analysis of randomized withdrawal trials. Semin. Arthritis Rheum. 46, 312–318 (2016).
Kerrigan, S. A. & McInnes, I. B. JAK inhibitors in rheumatology: implications for paediatric syndromes? Curr. Rheumatol. Rep. 20, 83–87 (2018).
Ruperto, N. et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet 398, 1984–1996 (2021).
Ramanan, A. V. et al. Baricitinib in juvenile idiopathic arthritis: an international, phase 3, randomised, double-blind, placebo-controlled, withdrawal, efficacy, and safety trial. Lancet 402, 555–570 (2023).
Brunner, H. I. et al. Safety and efficacy of upadacitinib for pediatric patients with polyarticular course juvenile idiopathic arthritis: an interim analysis of an open-label, phase 1 trial [abstract]. Ann. Rheum. Dis. 82, 108–109 (2023).
Qian, Y. et al. Pharmacokinetics of upadacitinib in pediatric patients with polyarticular course juvenile idiopathic arthritis [abstract]. Ann. Rheum. Dis. 82, 666–667 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03725007 (2023).
Ringold, S. et al. Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis. Arthritis Care. Res 66, 1063–1072 (2014).
Kimura, Y. et al. Optimizing the start time of biologics in polyarticular juvenile idiopathic arthritis: a comparative effectiveness study of Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans. Arthritis Rheumatol. 73, 1898–1909 (2021).
Ong, M. S. et al. Improved disease course associated with early initiation of biologics in polyarticular juvenile idiopathic arthritis: trajectory analysis of a Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans study. Arthritis Rheumatol. 73, 1910–1920 (2021).
Kimura, Y. et al. The Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA (STOP-JIA) study: three-year outcomes [abstract]. Arthritis Rheumatol. 74, (2022).
Weiss, P. F. et al. Children with enthesitis-related arthritis and possible benefits from treatments for adults with spondyloarthritis. Arthritis Care Res. 74, 1058–1064 (2022).
Chamlati, R. et al. Image guided sacroiliac joint corticosteroid injections in children: an 18-year single-center retrospective study. Pediatr. Rheumatol. Online J. 18, 52 (2020).
Oliver, M., Simard, J. F., Lee, T., Gerstbacher, D. & Sandborg, C. Determinants of tumor necrosis factor inhibitor use in juvenile spondyloarthropathy and impact on clinical disease outcomes. ACR Open. Rheumatol. 4, 19–26 (2022).
Burgos-Vargas, R. et al. A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis. Arthritis Res. Ther. 24, 187 (2022).
Horneff, G. et al. Efficacy and safety of etanercept in patients with the enthesitis-related arthritis category of juvenile idiopathic arthritis: results from a phase III randomized, double-blind study. Arthritis Rheumatol. 67, 2240–2249 (2015).
Foeldvari, I. et al. Etanercept treatment for extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis, or psoriatic arthritis: 6-year efficacy and safety data from an open-label trial. Arthritis Res. Ther. 21, 125 (2019).
Burgos-Vargas, R. et al. A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res. 67, 1503–1512 (2015).
Favalli, E. G. et al. Real-life 10-year retention rate of first-line anti-TNF drugs for inflammatory arthritides in adult- and juvenile-onset populations: similarities and differences. Clin. Rheumatol. 36, 1747–1755 (2017).
Gaur, P., Misra, R. & Aggarwal, A. Natural killer cells and gamma-delta T cells alterations in enthesitis related arthritis category of juvenile idiopathic arthritis. Clin. Immunol. 161, 163–169 (2015).
Mahendra, A., Misra, R. & Aggarwal, A. Th1 and Th17 predominance in enthesitis related arthritis form of juvenile idiopathic arthritis. J. Rheumatol. 36, 1730–1736 (2009).
Braun, J., Baraliakos, X. & Kiltz, U. Secukinumab (AIN457) in the treatment of ankylosing spondylitis. Expert. Opin. Biol. Ther. 16, 711–722 (2016).
Brunner, H. I. et al. Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann. Rheum. Dis. 82, 154–160 (2023).
Baer, J., Klotsche, J. & Foeldvari, I. Secukinumab in the treatment for patients with juvenile enthesitis related arthritis non-responsive to anti-TNF treatment according the Juvenile Spondyloarthritis Disease Activity Index. Clin. Exp. Rheumatol. 40, 620–624 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04527380 (2024).
Rahman, M. M., Laila, K. & Rahman, S. A. Efficacy and safety of tofacitinib in the treatment of refractory cases of polyarticular course juvenile idiopathic arthritis: a study from Bangladesh. Int. J. Rheum. Dis. 25, 678–684 (2022).
Stoll, M. L. & Mellins, E. D. Psoriatic arthritis in childhood: a commentary on the controversy. Clin. Immunol. 214, 108396 (2020).
Naddei, R. et al. Juvenile psoriatic arthritis: myth or reality? An unending debate. J. Clin. Med. 12, 367 (2023).
Ravelli, A., Consolaro, A., Schiappapietra, B. & Martini, A. The conundrum of juvenile psoriatic arthritis. Clin. Exp. Rheumatol. 33, S40–S43 (2015).
Constantin, T. et al. Two-year efficacy and safety of etanercept in pediatric patients with extended oligoarthritis, enthesitis-related arthritis, or psoriatic arthritis. J. Rheumatol. 43, 816–824 (2016).
Leu, J. H. et al. Intravenous golimumab in patients with polyarticular juvenile idiopathic arthritis and juvenile psoriatic arthritis and subcutaneous ustekinumab in patients with juvenile psoriatic arthritis: extrapolation of data from studies in adults and adjacent pediatric populations. Paediatr. Drugs 24, 699–714 (2022).
Wang, E. A., Suzuki, E., Maverakis, E. & Adamopoulos, I. E. Targeting IL-17 in psoriatic arthritis. Eur. J. Rheumatol. 4, 272–277 (2017).
Navarro-Compan, V. et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front. Immunol. 14, 1191782 (2023).
Schinocca, C. et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front. Immunol. 12, 637829 (2021).
Philipp, S. et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥6 to <12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br. J. Dermatol. 183, 664–672 (2020).
Landells, I. et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J. Am. Acad. Dermatol. 73, 594–603 (2015).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Halyabar, O., Mehta, J., Ringold, S., Rumsey, D. G. & Horton, D. B. Treatment withdrawal following remission in juvenile idiopathic arthritis: a systematic review of the literature. Paediatr. Drugs 21, 469–492 (2019).
Gerss, J. et al. Prevention of disease flares by risk-adapted stratification of therapy withdrawal in juvenile idiopathic arthritis: results from the PREVENT-JIA trial. Ann. Rheum. Dis. 81, 990–997 (2022).
Gieling, J., van den Bemt, B., Hoppenreijs, E. & Schatorjé, E. Discontinuation of biologic DMARDs in non-systemic JIA patients: a scoping review of relapse rates and associated factors. Pediatr. Rheumatol. Online J. 20, 109 (2022).
Ringold, S. et al. Disease recapture rates after medication discontinuation and flare in juvenile idiopathic arthritis: an observational study within the Childhood Arthritis and Rheumatology Research Alliance registry. Arthritis Care. Res. 75, 715–723 (2023).
Schanberg, L. E. et al. Therapeutic development in polyarticular course juvenile idiopathic arthritis: extrapolation, dose selection, and clinical trial design. Arthritis Rheumatol. 75, 1856–1866 (2023).
Burrone, M. et al. Looking for the best strategy to treat children with new onset juvenile idiopathic arthritis: presentation of the “comparison of STep-up and step-down therapeutic strategies in childhood ARthritiS” (STARS) trial. Pediatr. Rheumatol. Online J. 20, 80 (2022).
Wedderburn, L. R. et al. Towards molecular-pathology informed clinical trials in childhood arthritis to achieve precision medicine in juvenile idiopathic arthritis. Ann. Rheum. Dis. 82, 449–456 (2022).
Patient-Centered Outcomes Research Institute (PCORI). Trial of Sequential Medications after TNF Failure in Juvenile Idiopathic Arthritis (SMART-JIA). PCORI http://www.pcori.org/research-results/2023/trial-sequential-medications-after-tnf-failure-juvenile-idiopathic-arthritis-smart-jia#project_summary (2023).
Scott, C. et al. Juvenile arthritis management in less resourced countries (JAMLess): consensus recommendations from the Cradle of Humankind. Clin. Rheumatol. 38, 563–575 (2019).
Consolaro, A. et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child. Adolesc. Health 3, 255–263 (2019).
Slamang, W., Smith, N., Scott, C. & Foster, H. Revising the WHO Essential Medicines List for paediatric rheumatology update. Pediatr. Rheumatol. 20, 89 (2022).
Stoll, M. L. & Cron, R. Q. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr. Rheumatol. Online J. 12, 13 (2014).
Ahmed, W. et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20, e361–e379 (2022).
Record, J. L., Beukelman, T. & Cron, R. Q. Combination therapy of abatacept and anakinra in children with refractory systemic juvenile idiopathic arthritis: a retrospective case series. J. Rheumatol. 38, 180–181 (2011).
Dolinger, M. T., Spencer, E. A., Lai, J., Dunkin, D. & Dubinsky, M. C. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 27, 1210–1214 (2021).
Furer, V. & Elkayam, O. Dual biologic therapy in patients with rheumatoid arthritis and psoriatic arthritis. Rambam Maimonides Med. J. 14, e0007 (2023).
Brunner, H. I. et al. New medications are needed for children with juvenile idiopathic arthritis. Arthritis Rheumatol. 72, 1945–1951 (2020).
Bava, C. et al. Analysis of arthritis flares after achievement of inactive disease with methotrexate monotherapy in juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 39, 426–433 (2021).
Mannion, M. L. & Cron, R. Q. To wean or not to wean: that is the question. Arthritis Care Res. 75, 712–714 (2023).
Onel, K.B. et al. 2021 American College of Rheumatology Guideline for the treatment of juvenile idiopathic arthritis: recommendations for nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Arthritis Care Res. 74, 505–520 (2022).
Brunner, H. I. et al. Subcutaneous golimumab for children with active polyarticular-course juvenile idiopathic arthritis: results of a multicentre, double-blind, randomised withdrawal trial. Ann. Rheum. Dis. 77, 21–29 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05083182 (2024).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article
Corresponding author
Ethics declarations
Competing interests
S.S. declares that she has received consulting fees from Amgen, Novartis and Pfizer, all unrelated to this manuscript. G.H. declares that has he has received grants from MSD, Novartis, Pfizer, Roche and consulting and/or speaker’s fees from AbbVie, Boehringer, Celgene, Chugai, GSK, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi and Sobi, all unrelated to this manuscript. A.R. declares that has he has received grants from Novartis and Pfizer and consulting and/or speaker’s fees from AbbVie, Alexion, Angelini, Galapagos, Novartis, Pfizer, Reckitt-Benkiser, Roche, BMS and SOBI, all unrelated to this manuscript. A.A. declares no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Yukiko Kimura and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cluster Consortium: https://www.clusterconsortium.org.uk/
IMID-Bio-UK: https://www.gla.ac.uk/research/az/imid/
UCAN-Can DU: https://www.ucancandu.com
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shenoi, S., Horneff, G., Aggarwal, A. et al. Treatment of non-systemic juvenile idiopathic arthritis. Nat Rev Rheumatol 20, 170–181 (2024). https://doi.org/10.1038/s41584-024-01079-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01079-8
This article is cited by
-
Anti-tumor necrosis factor (aTNF) weaning strategy in juvenile idiopathic arthritis (JIA): does duration matter?
Clinical Rheumatology (2024)