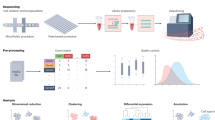
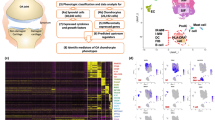
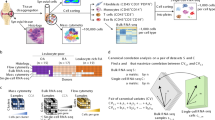
Abstract
Advances in the profiling of human joint tissues at single-cell resolution have provided unique insights into the organization and function of these tissues in health and disease. Data generated by various single-cell technologies, including single-cell RNA sequencing and cytometry by time-of-flight, have identified the distinct subpopulations that constitute these tissues. These timely studies have provided the building blocks for the construction of single-cell atlases of joint tissues including cartilage, bone and synovium, leading to the identification of developmental trajectories, deciphering of crosstalk between cells and discovery of rare populations such as stem and progenitor cells. In addition, these studies have revealed unique pathogenetic populations that are potential therapeutic targets. The use of these approaches in synovial tissues has helped to identify how distinct cell subpopulations can orchestrate disease initiation and progression and be responsible for distinct pathological outcomes. Additionally, repair of tissues such as cartilage and meniscus remains an unmet medical need, and single-cell methodologies can be invaluable in providing a blueprint for both effective tissue-engineering strategies and therapeutic interventions for chronic joint diseases such as osteoarthritis and rheumatoid arthritis.
Key points
-
Single-cell technologies (transcriptomic, proteomic and epigenomic) have seen considerable advancement in the past decade in elucidating the complexity and heterogeneity of cell populations that constitute joint tissues.
-
Studies using single-cell techniques have revealed the developmental trajectories of cell populations and their crosstalk.
-
Key cell populations with distinct pathogenetic or regenerative functions have been identified and can provide precise therapeutic targets for joint diseases.
-
Identification of pathogenetic cell populations provides insight into patient heterogeneity and cellular biomarkers for clinical stratification.
-
Single-cell data provide high-resolution snapshots of response to drugs in patient samples.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Cheung, P., Khatri, P., Utz, P. J. & Kuo, A. J. Single-cell technologies — studying rheumatic diseases one cell at a time. Nat. Rev. Rheumatol. 15, 340–354 (2019).
Aldridge, S. & Teichmann, S. A. Single cell transcriptomics comes of age. Nat. Commun. 11, 9–12 (2020).
Sebastian, A. et al. Single-cell RNA-seq reveals transcriptomic heterogeneity and post-traumatic osteoarthritis-associated early molecular changes in mouse articular chondrocytes. Cells 10, 1462 (2021).
Ji, Q. et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis. 78, 100–110 (2019).
Shen, P. & Löhning, M. Insights into osteoarthritis development from single-cell RNA sequencing of subchondral bone. RMD open. 8, 1–5 (2022).
Gong, Y. et al. A systematic dissection of human primary osteoblasts in vivo at single-cell resolution. Aging 13, 20629–20650 (2021).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Bian, Q. et al. A single cell transcriptional atlas of early synovial joint development. Development 147, dev185777 (2020).
Fu, W. et al. Cellular features of localized microenvironments in human meniscal degeneration: a single-cell transcriptomic study. Elife 11, 1–24 (2022).
Kouroupis, D., Best, T. M., Kaplan, L. D., Correa, D. & Griswold, A. J. Single-cell RNA-sequencing identifies infrapatellar fat pad macrophage polarization in acute synovitis/fat pad fibrosis and cell therapy. Bioengineering 8, 166 (2021).
Grandi, F. C. et al. Single-cell mass cytometry reveals cross-talk between inflammation-dampening and inflammation-amplifying cells in osteoarthritic cartilage. Sci. Adv. 6, 1–14 (2020).
Sahu, N., Grandi, F. C. & Bhutani, N. A single-cell mass cytometry platform to map the effects of preclinical drugs on cartilage homeostasis. JCI Insight 7, e160702 (2022).
Koppejan, H. et al. Immunoprofiling of early, untreated rheumatoid arthritis using mass cytometry reveals an activated basophil subset inversely linked to ACPA status. Arthritis Res. Ther. 23, 1–11 (2021).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Spitzer, M. H. & Nolan, G. P. Mass cytometry: single cells, many. features. Cell 165, 780–791 (2016).
Buenrostro, J., Wu, B., Chang, H. & Greenleaf, W. ATAC-seq method. Curr. Protoc. Mol. Biol. 2015, 1–10 (2016).
Guo, H. et al. The DNA methylation landscape of human early embryos. Nature 511, 606–610 (2014).
Clark, S. J. et al. Genome-wide base-resolution mapping of DNA methylation in single cells using single-cell bisulfite sequencing (scBS-seq). Nat. Protoc. 12, 534–547 (2017).
Armaka, M. et al. Single-cell multimodal analysis identifies common regulatory programs in synovial fibroblasts of rheumatoid arthritis patients and modeled TNF-driven arthritis. Genome Med. 14, 1–25 (2022).
Mow, V. C. & Lai, W. M. Mechanics of animal Joints. Annu. Rev. Fluid Mech. 11, 247–288 (1979).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 4, 1–23 (2018).
Alamanos, Y. & Drosos, A. A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 4, 130–136 (2005).
Chen, D. et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 5, 16044 (2017).
Man, G. S. & Mologhianu, G. Osteoarthritis pathogenesis — a complex process that involves the entire joint. J. Med. Life 7, 37–41 (2014).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6, 15 (2018).
Schett, G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 9, 1–6 (2007).
Takayanagi, H. RANKL as the master regulator of osteoclast differentiation. J. Bone Miner. Metab. 39, 13–18 (2021).
de Lange-Brokaar, B. J. E. et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 20, 1484–1499 (2012).
Takeuchi, Y., Hirota, K. & Sakaguchi, S. Synovial tissue inflammation mediated by autoimmune T cells. Front. Immunol. 10, 1–7 (2019).
Culemann, S. et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572, 670–675 (2019).
Jiang, Y. & Tuan, R. S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 11, 206–212 (2015).
Koelling, S. et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4, 324–335 (2009).
Ju, J. H. et al. CD24 enhances DNA damage-induced apoptosis by modulating NF-κB signaling in CD44-expressing breast cancer cells. Carcinogenesis 32, 1474–1483 (2011).
Lee, J., Smeriglio, P., Dragoo, J., Maloney, W. J. & Bhutani, N. CD24 enrichment protects while its loss increases susceptibility of juvenile chondrocytes towards inflammation. Arthritis Res. Ther. 18, 1–11 (2016).
Loeser, R. F. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 17, 971–979 (2009).
Jeon, O. H., David, N., Campisi, J. & Elisseeff, J. H. Senescent cells and osteoarthritis: a painful connection. J. Clin. Invest. 128, 1229–1237 (2018).
Liu, Y., Zhang, Z., Li, T., Xu, H. & Zhang, H. Senescence in osteoarthritis: from mechanism to potential treatment. Arthritis Res. Ther. 24, 1–15 (2022).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
D. Smith, M. The normal synovium. Open. Rheumatol. J. 5, 100–106 (2012).
Orr, C. et al. Synovial tissue research: a state-of-the-art review. Nat. Rev. Rheumatol. 13, 463–475 (2017).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 1–11 (2018).
Alivernini, S. et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 26, 1295–1306 (2020).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228 (2019).
Baryawno, N. et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell 177, 1915–1932.e16 (2019).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Dolgalev, I. & Tikhonova, A. N. Connecting the dots: resolving the bone marrow niche heterogeneity. Front. Cell Dev. Biol. 9, 1–11 (2021).
Chan, C. K. F. et al. Identification of the human skeletal stem cell. Cell 175, 43–56.e21 (2018).
Chan, C. K. F. et al. Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298 (2015).
Ambrosi, T. H., Longaker, M. T. & Chan, C. K. F. A revised perspective of skeletal stem cell biology. Front. Cell Dev. Biol. 7, 189 (2019).
Ambrosi, T. H. et al. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. Elife 10, 1–24 (2021).
Severe, N. et al. Stress-induced changes in bone marrow stromal cell populations revealed through single-cell protein expression mapping. Cell Stem Cell 25, 570–583.e7 (2019).
Yoshioka, H. et al. Single-cell RNA-sequencing reveals the breadth of osteoblast heterogeneity. JBMR Plus 5, 1–12 (2021).
Qiu, X. et al. Single-cell RNA sequencing of human femoral head in vivo. Aging 13, 15595–15619 (2021).
Hu, Y. et al. Single-cell RNA-sequencing analysis reveals the molecular mechanism of subchondral bone cell heterogeneity in the development of osteoarthritis. RMD Open. 8, 1–15 (2022).
Hu, Y., Chen, X., Wang, S., Jing, Y. & Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 9, 1–13 (2021).
Tsukasaki, M. et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab. 2, 1382–1390 (2020).
Zeng, N., Yan, Z. P., Chen, X. Y. & Ni, G. X. Infrapatellar fat pad and knee osteoarthritis. Aging Dis. 11, 1317–1328 (2020).
Edama, M. et al. Morphological characteristics of the infrapatellar fat pad. Sci. Rep. 12, 1–9 (2022).
Ioan-Facsinay, A. & Kloppenburg, M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res. Ther. 15, 225 (2013).
Sun, H. et al. Single-cell RNA-seq analysis identifies meniscus progenitors and reveals the progression of meniscus degeneration. Ann. Rheum. Dis. 408–417 https://doi.org/10.1136/annrheumdis-2019-215926 (2019).
Sun, H. et al. Single-cell RNA sequencing reveals the cell types heterogenicity of human discoid lateral meniscus cells. J. Cell. Physiol. 237, 2469–2477 (2022).
Udo, M. et al. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: proposed model-specific scoring systems. Osteoarthr. Cartil. 24, 1284–1291 (2016).
Inomata, K. et al. Time course analyses of structural changes in the infrapatellar fat pad and synovial membrane during inflammation-induced persistent pain development in rat knee joint. BMC Musculoskelet. Disord. 20, 1–10 (2019).
Takahashi, I., Matsuzaki, T., Kuroki, H. & Hoso, M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS One 13, 1–15 (2018).
Murphy, M. P. et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 26, 1583–1592 (2020).
Murphy, M. P., Koepke, L. S., Lopez, M. T., Tong, X. & Ambrosi, T. H. Articular cartilage regeneration by activated skeletal stem cells in mouse and human. Nat. Med. 26, 1582–1592 (2018).
Johnson, K. et al. A stem cell-based approach to cartilage repair. Science 336, 717–721 (2012).
Zhang, F. et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 623, 616–624 (2023).
Romão, V. C. & Pitzalis, C. Synovial heterogeneity in rheumatoid arthritis: the key for rational patient stratification? Acta Reumatol. Port. 2015, 6–8 (2015).
Robinson, W. H. & Mao, R. Biomarkers to guide clinical therapeutics in rheumatology? Curr. Opin. Rheumatol. 28, 168–175 (2016).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Bandura, D. R. et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009).
Shi, Q. et al. Single-cell proteomic chip for profiling intracellular signaling pathways in single tumor cells. Proc. Natl Acad. Sci. USA 109, 419–424 (2012).
Fu, W. et al. Cellular features of localized microenvironments in human meniscal degeneration: a single-cell transcriptomic study. eLife 11, e79585 (2022).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, 1360–1363 (2015).
Crosetto, N., Bienko, M. & Van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet. 16, 57–66 (2015).
Chou, C.-H. et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 10, 10868 (2020).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Cho, C. S. et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell 184, 3559–3572.e22 (2021).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Srivatsan, S. R. et al. Embryo-scale, single-cell spatial transcriptomics. Science 373, 111–117 (2021).
Vickovic, S. et al. Three-dimensional spatial transcriptomics uncovers cell type localizations in the human rheumatoid arthritis synovium. Commun. Biol. 5, 129 (2022).
Alon, S. et al. Expansion sequencing: spatially precise in situ transcriptomics in intact biological systems. Science 371, eaax2656 (2021).
Xia, C., Babcock, H. P., Moffitt, J. R. & Zhuang, X. Multiplexed detection of RNA using MERFISH and branched DNA amplification. Sci. Rep. 9, 1–13 (2019).
Kishi, J. Y. et al. Light-Seq: light-directed in situ barcoding of biomolecules in fixed cells and tissues for spatially indexed sequencing. Nat. Methods 19, 1393–1402 (2022).
Black, S. et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat. Protoc. 16, 3802–3835 (2021).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174, 968–981.e15 (2018).
Keren, L. et al. MIBI-TOF: a multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci. Adv. 5, 1–17 (2019).
van Dam, S., Baars, M. J. D. & Vercoulen, Y. Multiplex tissue imaging: spatial revelations in the tumor microenvironment. Cancers 14, 1–30 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Dana Orange, Kevin Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, A., Bhutani, N. Profiling joint tissues at single-cell resolution: advances and insights. Nat Rev Rheumatol 20, 7–20 (2024). https://doi.org/10.1038/s41584-023-01052-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01052-x