Abstract
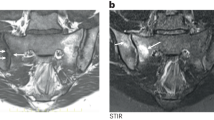
Since the original description of spondyloarthritis 50 years ago, results have demonstrated similarities and differences between ankylosing spondylitis (AS) and axial psoriatic arthritis (PsA). HLA-B27 gene carriage in axial inflammation is linked to peri-fibrocartilaginous sacroiliac joint osteitis, as well as to spinal peri-entheseal osteitis, which is often extensive and which provides a crucial anatomical and immunological differentiation between the AS and PsA phenotypes. Specifically, HLA-B27-related diffuse bone marrow oedema (histologically an osteitis) and bone marrow fatty corners detected via magnetic resonance imaging, as well as radiographic changes such as sacroiliitis, vertebral squaring, corner erosions and Romanus lesions, all indicate initial bone phenotypes in HLA-B27+ axial disease. However, in much of PsA with axial involvement, enthesitis primarily manifests in ligamentous soft tissue as ‘ligamentitis’, with characteristic lesions that include para-syndesmophytes and sacroiliac joint bony sparing. Like axial PsA, diffuse idiopathic skeletal hyperostosis phenotypes, which can be indistinguishable from PsA, exhibit a thoracic and cervical spinal ligamentous soft-tissue tropism, clinically manifesting as syndesmophytosis that is soft-tissue-centric, including paravertebral soft-tissue ossification and sacroiliac soft-ligamentous ossification instead of joint-cavity fusion. The enthesis bone and soft tissues have radically different immune cell and stromal compositions, which probably underpins differential responses to immunomodulatory therapy, especially IL-23 inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Moll, J. M. & Wright, V. Psoriatic arthritis. Semin. Arthritis Rheum. 3, 55–78 (1973).
Jadon, D. R. et al. Axial disease in psoriatic arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann. Rheum. Dis. 76, 701–707 (2017).
Feld, J. et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology 59, 1340–1346 (2020).
Kwok, T. S. H. et al. Isolated axial disease in psoriatic arthritis and ankylosing spondylitis with psoriasis. Ann. Rheum. Dis. 81, 1678–1684 (2022).
Baeten, D. et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 77, 1295–1302 (2018).
Deodhar, A. et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 71, 258–270 (2019).
Siebert, S., Millar, N. L. & McInnes, I. B. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann. Rheum. Dis. 78, 1015–1018 (2019).
McGonagle, D., Watad, A., Sharif, K. & Bridgewood, C. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front. Immunol. 12, 614255 (2021).
Deodhar, A. et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 395, 1115–1125 (2020).
Mease, P. J. et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 395, 1126–1136 (2020).
Ramiro, S. et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 82, 19 (2023).
Coates, L. C. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 18, 465–479 (2022).
Sieper, J. et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann. Rheum. Dis. 68, 784–788 (2009).
Bennett, A. N. et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum. 58, 3413–3418 (2008).
Haddad, A., Thavaneswaran, A., Toloza, S., Chandran, V. & Gladman, D. D. Diffuse idiopathic skeletal hyperostosis in psoriatic arthritis. J. Rheumatol. 40, 1367–1373 (2013).
Gladman, D. D. Axial psoriatic arthritis. Curr. Rheumatol. Rep. 23, 35 (2021).
Poddubnyy, D. et al. Axial Involvement in Psoriatic Arthritis cohort (AXIS): the protocol of a joint project of the Assessment of SpondyloArthritis international Society (ASAS) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Ther. Adv. Musculoskelet. Dis. 13, 1759720X211057975 (2021).
Benjamin, M. & McGonagle, D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat. 199, 503–526 (2001).
Bollow, M. et al. Early sacroiliitis in patients with spondyloarthropathy: evaluation with dynamic gadolinium-enhanced MR imaging. Radiology 194, 529–536 (1995).
Hermann, K. G. & Bollow, M. Magnetic resonance imaging of sacroiliitis in patients with spondyloarthritis: correlation with anatomy and histology. RoFo 186, 230–237 (2014).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Ridley, L. K. et al. Why do some patients have severe sacroiliac disease but no syndesmophytes in ankylosing spondylitis? Data from a nested case-control study. J. Rheumatol. 50, 335–341 (2022).
Oostveen, J., Prevo, R., den Boer, J. & van de Laar, M. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography. A prospective, longitudinal study. J. Rheumatol. 26, 1953–1958 (1999).
Puhakka, K. B. et al. Magnetic resonance imaging of sacroiliitis in early seronegative spondylarthropathy. Abnormalities correlated to clinical and laboratory findings. Rheumatology 43, 234–237 (2004).
Bollow, M. et al. Very early spondyloarthritis: where the inflammation in the sacroiliac joints starts. Ann. Rheum. Dis. 64, 1644 (2005).
Appel, H. et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res. Ther. 8, R143 (2006).
Marzo-Ortega, H., O’Connor, P., Emery, P. & McGonagle, D. Sacroiliac joint biopsies in early sacroiliitis. Rheumatology 46, 1210–1211 (2007).
Gracey, E. et al. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 16, 193–207 (2020).
Romero-López, J. P., Elewaut, D., Pacheco-Tena, C. & Burgos-Vargas, R. Inflammatory foot involvement in spondyloarthritis: from tarsitis to ankylosing tarsitis. Front. Med. 8, 730273 (2021).
Pacheco-Tena, C. et al. Bone lineage proteins in the entheses of the midfoot in patients with spondyloarthritis. J. Rheumatol. 42, 630–637 (2015).
Marzo-Ortega, H. et al. Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann. Rheum. Dis. 68, 1721–1727 (2009).
McGonagle, D. et al. The role of biomechanical factors and HLA-B27 in magnetic resonance imaging-determined bone changes in plantar fascia enthesopathy. Arthritis Rheum. 46, 489–493 (2002).
Lambert, R. G. W. et al. High prevalence of symptomatic enthesopathy of the shoulder in ankylosing spondylitis: deltoid origin involvement constitutes a hallmark of disease. Arthritis Care Res. 51, 681–690 (2004).
Braun, J., Bollow, M. & Sieper, J. Radiologic diagnosis and pathology of the spondyloarthropathies. Rheum. Dis. Clin. North Am. 24, 697–735 (1998).
Baraliakos, X. et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. Ann. Rheum. Dis. 79, 186–192 (2020).
Caetano, A. P., Mascarenhas, V. V. & Machado, P. M. Axial spondyloarthritis: mimics and pitfalls of imaging assessment. Front. Med. 8, 658538 (2021).
Baraliakos, X., Listing, J., Rudwaleit, M., Sieper, J. & Braun, J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res. Ther. 10, R104 (2008).
Ball, J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann. Rheum. Dis. 30, 213–223 (1971).
Chung, H. Y., Yiu, R. S. W., Chan, S. C. W., Lee, K. H. & Lau, C. S. Fatty corner lesions in T1-weighted magnetic resonance imaging as an alternative to sacroiliitis for diagnosis of axial spondyloarthritis. BMC Rheumatol. 3, 17 (2019).
Bakker, P. A. C. et al. Can we use structural lesions seen on MRI of the sacroiliac joints reliably for the classification of patients according to the ASAS axial spondyloarthritis criteria? Data from the DESIR cohort. Ann. Rheum. Dis. 76, 392–398 (2017).
Baraliakos, X. et al. What constitutes the fat signal detected by MRI in the spine of patients with ankylosing spondylitis? A prospective study based on biopsies obtained during planned spinal osteotomy to correct hyperkyphosis or spinal stenosis. Ann. Rheum. Dis. 78, 1220–1225 (2019).
Bennett, A. N. et al. The fatty Romanus lesion: a non-inflammatory spinal MRI lesion specific for axial spondyloarthropathy. Ann. Rheum. Dis. 69, 891–894 (2010).
Castillo-Gallego, C., Aydin, S. Z., Emery, P., McGonagle, D. G. & Marzo-Ortega, H. Brief report: magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA–B27. Arthritis Rheum. 65, 2274–2278 (2013).
Baraliakos, X. et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann. Rheum. Dis. 80, 582–590 (2021).
Michelena, X. et al. Characterising the axial phenotype of psoriatic arthritis: a study comparing axial psoriatic arthritis and ankylosing spondylitis with psoriasis from the REGISPONSER registry. RMD Open 8, e002513 (2022).
Castillo-Gallego, C., Aydin, S. Z., Emery, P., McGonagle, D. G. & Marzo-Ortega, H. Magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA-B27. Arthritis Rheum. 65, 2274–2278 (2013).
Marzo-Ortega, H., McGonagle, D., O’Connor, P. & Emery, P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum. 44, 2112–2117 (2001).
Rudwaleit, M. et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 68, 1520–1527 (2009).
Baraliakos, X. et al. MRI lesions of the spine in patients with axial spondyloarthritis: an update of lesion definitions and validation by the ASAS MRI working group. Ann. Rheum. Dis. 81, 1243 (2022).
Mauro, D. et al. The bone marrow side of axial spondyloarthritis. Nat. Rev. Rheumatol. 19, 519–532 (2023).
Maksymowych, W. P. Evidence in support of the bone marrow as the primary lesion in axial spondyloarthritis. Curr. Opin. Rheumatol. 35, 213–218 (2023).
Cortes, A. et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat. Commun. 6, 7146 (2015).
McGonagle, D., Aydin, S. Z., Gül, A., Mahr, A. & Direskeneli, H. ‘MHC-I-opathy’-unified concept for spondyloarthritis and Behçet disease. Nat. Rev. Rheumatol. 11, 731–740 (2015).
Nair, R. P. et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet 78, 827–851 (2006).
Feltkamp, T. E. Ophthalmological significance of HLA associated uveitis. Eye 4, 839–844 (1990).
Winchester, R. et al. HLA associations reveal genetic heterogeneity in psoriatic arthritis and in the psoriasis phenotype. Arthritis Rheum. 64, 1134–1144 (2012).
Kuiper, J. J. et al. EULAR study group on ‘MHC-I-opathy’: identifying disease-overarching mechanisms across disciplines and borders. Ann. Rheum. Dis. 82, 887–896 (2023).
Díaz-Peña, R., Castro-Santos, P., Durán, J., Santiago, C. & Lucia, A. The genetics of spondyloarthritis. J. Pers. Med. 10, 151 (2020).
Eder, L. et al. Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 71, 50–55 (2012).
Ritchlin, C. T., Colbert, R. A. & Gladman, D. D. Psoriatic arthritis. N. Engl. J. Med. 376, 957–970 (2017).
Timothy, S. H. K. et al. Isolated axial disease in psoriatic arthritis and ankylosing spondylitis with psoriasis. Ann. Rheum. Dis. 81, 1678 (2022).
Savage, L. et al. Regression of peripheral subclinical enthesopathy in therapy-naive patients treated with ustekinumab for moderate-to-severe chronic plaque psoriasis: a fifty-two-week, prospective, open-label feasibility study. Arthritis Rheumatol. 71, 626–631 (2019).
Loeser, R. F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin. Geriat. Med. 26, 371–386 (2010).
Jurik, A. G. Imaging the spine in arthritis—a pictorial review. Insights Imaging 2, 177–191 (2011).
Feld, J. et al. Axial disease in psoriatic arthritis: the presence and progression of unilateral grade 2 sacroiliitis in a psoriatic arthritis cohort. Semin. Arthritis Rheum. 51, 464–468 (2021).
Renson, T. et al. Progressive increase in sacroiliac joint and spinal lesions detected on magnetic resonance imaging in healthy individuals in relation to age. Arthritis Rheumatol. 74, 1506–1514 (2022).
Sherratt, M. J. Age-related tissue stiffening: cause and effect. Adv. Wound Care 2, 11–17 (2013).
Tan, A. L. et al. Combined high-resolution magnetic resonance imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann. Rheum. Dis. 65, 1267–1272 (2006).
Tan, A. L. et al. High-resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum. 52, 2355–2365 (2005).
Tan, A. L., Grainger, A. J., Tanner, S. F., Emery, P. & McGonagle, D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum. 54, 1328–1333 (2006).
Braum, L. S. et al. Characterisation of hand small joints arthropathy using high-resolution MRI—limited discrimination between osteoarthritis and psoriatic arthritis. Eur. Radio. 23, 1686–1693 (2013).
Altman, R. et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 29, 1039–1049 (1986).
Waghray, N., Jyothi, G. A., Imran, M., Yaseen, S. & Chaudhary, U. Enthesis: a brief review. Apollo Med. 12, 32–38 (2015).
Urban, J. P. & Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 5, 120–130 (2003).
Ziegeler, K., Eshed, I., Diekhoff, T. & Hermann, K. G. Imaging of joints and bones in autoinflammation. J. Clin. Med. 9, 4074 (2020).
Eshed, I. et al. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann. Rheum. Dis. 66, 1553–1559 (2007).
Benjamin, M. & McGonagle, D. Histopathologic changes at “synovio–entheseal complexes” suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 56, 3601–3609 (2007).
Mathew, A. J. & Østergaard, M. Magnetic resonance imaging of enthesitis in spondyloarthritis, including psoriatic arthritis—status and recent advances. Front. Med. 7, 296 (2020).
McGonagle, D., Palmou Fontana, N., Tan, A. L. & Benjamin, M. Nailing down the genetic and immunological basis for psoriatic disease. Dermatology 221, 15–22 (2010).
Baraliakos, X. et al. Predictors of response to secukinumab in patients with psoriatic arthritis and axial manifestations: a post-hoc analysis of the MAXIMISE trial. RMD Open 8, e002303 (2022).
Mader, R., Verlaan, J. J. & Buskila, D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat. Rev. Rheumatol. 9, 741–750 (2013).
McGonagle, D., Hermann, K. G. & Tan, A. L. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology 54, 29–38 (2015).
Eshed, I. Imaging characteristics of diffuse idiopathic skeletal hyperostosis: more than just spinal bony bridges. Diagnostics 13, 563 (2023).
Latourte, A. et al. Imaging findings suggestive of axial spondyloarthritis in diffuse idiopathic skeletal hyperostosis. Arthritis Care Res. 70, 145–152 (2018).
Mader, R. et al. Imaging of diffuse idiopathic skeletal hyperostosis (DISH). RMD Open 6, e001151 (2020).
Hochberg, M. C., Borenstein, D. G. & Arnett, F. C. The absence of back pain in classical ankylosing spondylitis. Johns Hopkins Med. J. 143, 181–183 (1978).
Littlejohn, G. O. Insulin and new bone formation in diffuse idiopathic skeletal hyperostosis. Clin. Rheumatol. 4, 294–300 (1985).
Youssef, A., Aboalola, D. & Han, V. K. M. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells Int. 2017, 9453108 (2017).
Crane, J. L. et al. IGF-1 signaling is essential for differentiation of mesenchymal stem cells for peak bone mass. Bone Res. 1, 186–194 (2013).
Riley, R. S. et al. Bone marrow aspirate and biopsy: a pathologist’s perspective. II. Interpretation of the bone marrow aspirate and biopsy. J. Clin. Lab. Anal. 23, 259–307 (2009).
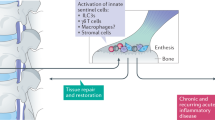
Macleod, T., Bridgewood, C. & McGonagle, D. Role of neutrophil interleukin-23 in spondyloarthropathy spectrum disorders. Lancet Rheumatol. 5, e47–e57 (2023).
Bridgewood, C. et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann. Rheum. Dis. 78, 929–933 (2019).
Stavre, Z. et al. A role for neutrophils in early enthesitis in spondyloarthritis. Arthritis Res. Ther. 24, 24 (2022).
Coletto, L. A. et al. The role of neutrophils in spondyloarthritis: a journey across the spectrum of disease manifestations. Int. J. Mol. Sci. 24, 4108 (2023).
Bridgewood, C., Sharif, K., Sherlock, J., Watad, A. & McGonagle, D. Interleukin-23 pathway at the enthesis: the emerging story of enthesitis in spondyloarthropathy. Immunol. Rev. 294, 27–47 (2020).
Sunwoo, J. Y., Eliasberg, C. D., Carballo, C. B. & Rodeo, S. A. The role of the macrophage in tendinopathy and tendon healing. J. Orthop. Res. 38, 1666–1675 (2020).
Moeed, A. et al. Single cell and spatial transcriptomics in human tendon disease indicate dysregulated immune homeostasis. Ann. Rheum. Dis. 80, 1494–1497 (2021).
Gladman, D. D. et al. Efficacy and safety of guselkumab in biologic-naïve patients with active axial psoriatic arthritis: study protocol for STAR, a phase 4, randomized, double-blinded, placebo-controlled trial. Trials 23, 743 (2022).
Coates, L. C. et al. The phenotype of axial spondyloarthritis: is it dependent on HLA-B27 status? Arthritis Care Res. 73, 856–860 (2021).
Helliwell, P. S. Axial disease in psoriatic arthritis. Rheumatology 59, 1193–1195 (2020).
Helliwell, P. S., Hickling, P. & Wright, V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann. Rheum. Dis. 57, 135–140 (1998).
Acknowledgements
D.M.’s work is partially funded by the Leeds Biomedical Research Centre and Versus Arthritis.
Author information
Authors and Affiliations
Contributions
D.M. developed the concept. All authors contributed substantially to discussion of the content. D.M. wrote the first draft of the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.M. has received grant support from Abbvie, BMS, Janssen, Lilly, Novartis and UCB. A.W. has received grant support from Janssen. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Clementina Lopez-Medina, Victoria Navarro-Compán and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McGonagle, D., David, P., Macleod, T. et al. Predominant ligament-centric soft-tissue involvement differentiates axial psoriatic arthritis from ankylosing spondylitis. Nat Rev Rheumatol 19, 818–827 (2023). https://doi.org/10.1038/s41584-023-01038-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01038-9