Abstract
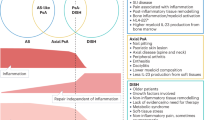
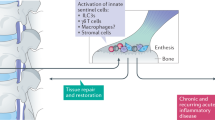
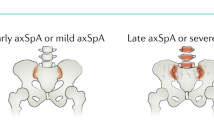
Spondyloarthritis (SpA) is characterized by the infiltration of innate and adaptive immune cells into entheses and bone marrow. Molecular, cellular and imaging evidence demonstrates the presence of bone marrow inflammation, a hallmark of SpA. In the spine and the peripheral joints, bone marrow is critically involved in the pathogenesis of SpA. Evidence suggests that bone marrow inflammation is associated with enthesitis and that there are roles for mechano-inflammation and intestinal inflammation in bone marrow involvement in SpA. Specific cell types (including mesenchymal stem cells, innate lymphoid cells and γδ T cells) and mediators (Toll-like receptors and cytokines such as TNF, IL-17A, IL-22, IL-23, GM-CSF and TGFβ) are involved in these processes. Using this evidence to demonstrate a bone marrow rather than an entheseal origin for SpA could change our understanding of the disease pathogenesis and the relevant therapeutic approach.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Robinson, P. C., van der Linden, S., Khan, M. A. & Taylor, W. J. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat. Rev. Rheumatol. 17, 109–118 (2020).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
van der Heijde, D. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991 (2017).
Mauro, D. et al. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat. Rev. Rheumatol. 17, 387–404 (2021).
Schett, G. et al. Psoriatic arthritis from a mechanistic perspective. Nat. Rev. Rheumatol. 18, 311–325 (2022).
Aydin, S. Z., Bridgewood, C., Zabotti, A., Girolimetto, N. & McGonagle, D. The transition from enthesis physiological responses in health to aberrant responses that underpin spondyloarthritis mechanisms. Curr. Opin. Rheumatol. 33, 64–73 (2020).
Jacques, P. et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis. 73, 437–445 (2014).
Schett, G. et al. Enthesitis: from pathophysiology to treatment. Nat. Rev. Rheumatol. 13, 731–741 (2017).
Ball, J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann. Rheum. Dis. 30, 213–223 (1971).
McGonagle, D., Lories, R. J. U., Tan, A. L. & Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 56, 2482–2491 (2007).
Sharif, K., Bridgewood, C., Dubash, S. & McGonagle, D. Intestinal and enthesis innate immunity in early axial spondyloarthropathy. Rheumatology 59, iv67–iv78 (2020).
Russell, T. et al. Cytokine ‘fine tuning’ of enthesis tissue homeostasis as a pointer to spondyloarthritis pathogenesis with a focus on relevant TNF and IL-17 targeted therapies. Semin. Immunopathol. 43, 193–206 (2021).
Weber, U. et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “background noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol. 70, 736–745 (2018).
Varkas, G. et al. Effect of mechanical stress on magnetic resonance imaging of the sacroiliac joints: assessment of military recruits by magnetic resonance imaging study. Rheumatology 57, 508–513 (2018).
Renson, T. et al. High prevalence of spondyloarthritis-like MRI lesions in postpartum women: a prospective analysis in relation to maternal, child and birth characteristics. Ann. Rheum. Dis. 79, 929–934 (2020).
Zubler, V., Agten, C. A., Pfirrmann, C. W. A., Weiss, B. G. & Dietrich, T. J. Frequency of arthritis-like MRI findings in the forefeet of healthy volunteers versus patients with symptomatic rheumatoid arthritis or psoriatic arthritis. AJR Am. J. Roentgenol. 208, W45–W53 (2017).
Mauro, D., Simone, D., Bucci, L. & Ciccia, F. Novel immune cell phenotypes in spondyloarthritis pathogenesis. Semin. Immunopathol. 43, 265–277 (2021).
Gracey, E. et al. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 16, 193–207 (2020).
Debusschere, K., Cambré, I., Gracey, E. & Elewaut, D. Born to run: the paradox of biomechanical force in spondyloarthritis from an evolutionary perspective. Best Pract. Res. Clin. Rheumatol. 31, 887–894 (2017).
Jadon, D. R., Stober, C., Pennington, S. R. & FitzGerald, O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat. Rev. Rheumatol. 16, 609–627 (2020).
Ciccia, F. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965 (2009).
Bridgewood, C. et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann. Rheum. Dis. 78, 929–933 (2019).
Mauro, D., Nakamura, A., Haroon, N. & Ciccia, F. The gut-enthesis axis and the pathogenesis of spondyloarthritis. Semin. Immunol. 58, 101607 (2021).
Gracey, E. et al. Revisiting the gut–joint axis: links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 16, 415–433 (2020).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Cuthbert, R. J. et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol. 69, 1816–1822 (2017).
Bridgewood, C., Sharif, K., Sherlock, J., Watad, A. & McGonagle, D. Interleukin‐23 pathway at the enthesis: the emerging story of enthesitis in spondyloarthropathy. Immunol. Rev. 294, 27–47 (2020).
Cuthbert, R. J. et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann. Rheum. Dis. 78, 1559–1565 (2019).
Rosine, N. et al. Characterization of blood mucosal-associated invariant T cells in patients with axial spondyloarthritis and of resident mucosal-associated invariant T cells from the axial entheses of non-axial spondyloarthritis control patients. Arthritis Rheumatol. 74, 1786–1795 (2022).
Watad, A. et al. Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann. Rheum. Dis. 79, 1044–1054 (2020).
Reinhardt, A. et al. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 68, 2476–2486 (2016).
Zhang, J. & Wang, J. H.-C. Production of PGE2 increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J. Orthop. Res. 28, 198–203 (2010).
Mauro, D. et al. Prostaglandin E2/EP4 axis is upregulated in spondyloarthritis and contributes to radiographic progression. Clin. Immunol. 251, 109332 (2023).
Wanders, A. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 52, 1756–1765 (2005).
Dubash, S. et al. Emergence of severe spondyloarthropathy-related entheseal pathology following successful vedolizumab therapy for inflammatory bowel disease. Rheumatology 58, 963–968 (2019).
Wang, C., Hanly, E., McDonald, K. & Newberry, R. The effect of a4β7 blockade on T-lymphocyte trafficking to intestinal compartments in the normal and inflamed intestine. Inflamm. Bowel Dis. 15, S47–S48 (2009).
Guggino, G., Rizzo, A., Mauro, D., Macaluso, F. & Ciccia, F. Gut-derived CD8+ tissue-resident memory T cells are expanded in the peripheral blood and synovia of SpA patients. Ann. Rheum. Dis. 80, e174 (2021).
Vanhoenacker, F. M. & Snoeckx, A. Bone marrow edema in sports: general concepts. Eur. J. Radiol. 62, 6–15 (2007).
Kornaat, P. R., de Jonge, M. C. & Maas, M. Bone marrow edema-like signal in the athlete. Eur. J. Radiol. 67, 49–53 (2008).
Kornaat, P. R. & van de Velde, S. K. Bone marrow edema lesions in the professional runner. Am. J. Sports Med. 42, 1242–1246 (2014).
de Cata, A., Inglese, M., Rubino, R., Molinaro, F. & Mazzoccoli, G. The synovio-entheseal complex in enthesoarthritis. Clin. Exp. Med. 16, 109–124 (2016).
Watad, A., Cuthbert, R. J., Amital, H. & McGonagle, D. Enthesitis: much more than focal insertion point inflammation. Curr. Rheumatol. Rep. 20, 41 (2018).
François, R. J., Braun, J. & Khan, M. A. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr. Opin. Rheumatol. 13, 255–264 (2001).
Maksymowych, W. P. The role of imaging in the diagnosis and management of axial spondyloarthritis. Nat. Rev. Rheumatol. 15, 657–672 (2019).
Lorenzin, M. et al. Spine and sacroiliac joints lesions on magnetic resonance imaging in early axial-spondyloarthritis during 24-months follow-up (Italian arm of SPACE study). Front. Immunol. 11, 936 (2020).
Maksymowych, W. P. et al. Central reader evaluation of MRI scans of the sacroiliac joints from the ASAS classification cohort: discrepancies with local readers and impact on the performance of the ASAS criteria. Ann. Rheum. Dis. 79, 935–942 (2020).
Maksymowych, W. P. et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann. Rheum. Dis. 78, 1550–1558 (2019).
Weber, U. et al. Does evaluation of the ligamentous compartment enhance diagnostic utility of sacroiliac joint MRI in axial spondyloarthritis? Arthritis Res. Ther. 17, 246 (2015).
Cruickshank, B. Pathology of ankylosing spondylitis. Bull. Rheum. Dis. 10, 211–214 (1960).
No authors listed. Clinicopathological conference. A case of early ankylosing spondylitis with fatal secondary amyloidosis. Br. Med. J. 2, 412–416 (1968).
Appel, H. et al. Immunohistochemical analysis of hip arthritis in ankylosing spondylitis: evaluation of the bone–cartilage interface and subchondral bone marrow. Arthritis Rheum. 54, 1805–1813 (2006).
Appel, H. et al. Immunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitis. Arthritis Rheum. 54, 2845–2851 (2006).
Bleil, J. et al. Histomorphologic and histomorphometric characteristics of zygapophyseal joint remodeling in ankylosing spondylitis. Arthritis Rheumatol. 66, 1745–1754 (2014).
Bleil, J. et al. Granulation tissue eroding the subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheumatol. 68, 2456–2465 (2016).
Pacheco-Tena, C. et al. Bone lineage proteins in the entheses of the midfoot in patients with spondyloarthritis. J. Rheumatol. 42, 630–637 (2015).
Muche, B. et al. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: a detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 48, 1374–1384 (2003).
Castillo-Gallego, C., Aydin, S. Z., Emery, P., McGonagle, D. G. & Marzo-Ortega, H. Brief report: magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA-B27. Arthritis Rheum. 65, 2274–2278 (2013).
McGonagle, D. et al. The role of biomechanical factors and HLA-B27 in magnetic resonance imaging-determined bone changes in plantar fascia enthesopathy. Arthritis Rheum. 46, 489–493 (2002).
Dougados, M. et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 68, 1904–1913 (2016).
Bollow, M. et al. Very early spondyloarthritis: where the inflammation in the sacroiliac joints starts. Ann. Rheum. Dis. 64, 1644–1666 (2005).
Shichikawa, K., Tsujimoto, M., Nishioka, J. & Matsumoto, K. in Advances in Inflammation Research: The Spondyloarthropathies Vol. 9 (eds Ziff, M. & Cohen, S. B.) 15–24 (Raven, 1985).
Wang, D. M. et al. Pannus inflammation in sacroiliitis following immune pathological injury and radiological structural damage: a study of 193 patients with spondyloarthritis. Arthritis Res. Ther. 20, 120 (2018).
Bollow, M. et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis – cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann. Rheum. Dis. 59, 135–140 (2000).
Dougados, M. et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann. Rheum. Dis. 76, 1823–1828 (2017).
Bennett, A. N. et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum. 58, 3413–3418 (2008).
Sepriano, A. et al. Is active sacroiliitis on MRI associated with radiographic damage in axial spondyloarthritis? Real-life data from the ASAS and DESIR cohorts. Rheumatology 58, 798–802 (2019).
Weber, U. et al. Can erosions on MRI of the sacroiliac joints be reliably detected in patients with ankylosing spondylitis? A cross-sectional study. Arthritis Res. Ther. 14, R124 (2012).
Maksymowych, W. P., Wichuk, S., Chiowchanwisawakit, P., Lambert, R. G. & Pedersen, S. J. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol. 66, 2958–2967 (2014).
Maksymowych, W. P., Wichuk, S., Chiowchanwisawakit, P., Lambert, R. G. & Pedersen, S. J. Fat metaplasia on MRI of the sacroiliac joints increases the propensity for disease progression in the spine of patients with spondyloarthritis. RMD Open 3, e000399 (2017).
Machado, P. M., Baraliakos, X., van der Heijde, D., Braun, J. & Landewé, R. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann. Rheum. Dis. 75, 1486–1493 (2016).
Baraliakos, X. et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann. Rheum. Dis. 73, 1819–1825 (2014).
Chiowchanwisawakit, P., Lambert, R. G. W., Conner-Spady, B. & Maksymowych, W. P. Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum. 63, 2215–2225 (2011).
Thomopoulos, S., Genin, G. M. & Galatz, L. M. The development and morphogenesis of the tendon-to-bone insertion – what development can teach us about healing. J. Musculoskelet. Neuronal Interact. 10, 35–45 (2010).
Rossetti, L. et al. The microstructure and micromechanics of the tendon–bone insertion. Nat. Mater. 16, 664–670 (2017).
Genin, G. M. et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J. 97, 976–985 (2009).
Killian, M. L. Growth and mechanobiology of the tendon–bone enthesis. Semin. Cell Dev. Biol. 123, 64–73 (2022).
Laloux, L. Immunohistological study of entheses in spondyloarthropathies: comparison in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 60, 316–321 (2001).
Benjamin, M. et al. Microdamage and altered vascularity at the enthesis–bone interface provides an anatomic explanation for bone involvement in the HLA-B27-associated spondylarthritides and allied disorders. Arthritis Rheum. 56, 224–233 (2007).
Grüneboom, A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 1, 236–250 (2019).
François, R. J., Neure, L., Sieper, J. & Braun, J. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor α in two patients with early disease and transforming growth factor β in three more advanced cases. Ann. Rheum. Dis. 65, 713–720 (2006).
Stavre, Z. et al. A role for neutrophils in early enthesitis in spondyloarthritis. Arthritis Res. Ther. 24, 24 (2022).
Macleod, T., Bridgewood, C. & McGonagle, D. Role of neutrophil interleukin-23 in spondyloarthropathy spectrum disorders. Lancet Rheumatol. 5, e47–e57 (2023).
Ciccia, F. et al. Proinflammatory CX3CR1+CD59+ tumor necrosis factor-like molecule 1A+interleukin-23+ monocytes are expanded in patients with ankylosing spondylitis and modulate innate lymphoid cell 3 immune functions. Arthritis Rheumatol. 70, 2003–2013 (2018).
Ciccia, F. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747 (2015).
Subramanian, A. & Schilling, T. F. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development 142, 4191–4204 (2015).
Zhen, G. et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712 (2013).
Das, M., Ithychanda, S., Qin, J. & Plow, E. F. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim. Biophys. Acta 1838, 579–588 (2014).
Zhen, G. et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 12, 1706 (2021).
Wang, X. et al. Aberrant TGF-β activation in bone tendon insertion induces enthesopathy-like disease. J. Clin. Invest. 128, 846–860 (2018).
Armaka, M. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 205, 331–337 (2008).
Adkisson, H. D. et al. Immune evasion by neocartilage-derived chondrocytes: implications for biologic repair of joint articular cartilage. Stem Cell Res. 4, 57–68 (2010).
Braun, J. et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 38, 499–505 (1995).
Appel, H. et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res. Ther. 8, R143 (2006).
Yang, H. K. et al. Regression of syndesmophyte after bone marrow transplantation for acute myeloid leukemia in a patient with ankylosing spondylitis: a case report. J. Med. Case Rep. 6, 250 (2012).
Britanova, O. V. et al. First autologous hematopoietic SCT for ankylosing spondylitis: a case report and clues to understanding the therapy. Bone Marrow Transplant. 47, 1479–1481 (2012).
Simonetta, F. et al. Complete and sustained remission of spondyloarthritis after allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome. Jt. Bone Spine 82, 216–217 (2014).
Taugor, J. D. et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol. Rev. 169, 209–223 (1999).
Breban, M., Hammer, R. E., Richardson, J. A. & Taurog, J. D. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J. Exp. Med. 178, 1607–1616 (1993).
Wang, R. & Maksymowych, W. P. Targeting the interleukin-23/interleukin-17 inflammatory pathway: successes and failures in the treatment of axial spondyloarthritis. Front. Immunol. 12, 3472 (2021).
Appel, H. et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. 65, 1522–1529 (2013).
Mauro, D., Macaluso, F., Fasano, S., Alessandro, R. & Ciccia, F. ILC3 in axial spondyloarthritis: the gut angle. Curr. Rheumatol. Rep. 21, 37 (2019).
Toussirot, É., Laheurte, C., Gaugler, B., Gabriel, D. & Saas, P. Increased IL-22-and IL-17A-producing mucosal-associated invariant T cells in the peripheral blood of patients with ankylosing spondylitis. Front. Immunol. 9, 1610 (2018).
Sonnenberg, G. F., Fouser, L. A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 (2011).
El-Zayadi, A. A. et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology 56, 488–493 (2016).
Slouma, M. et al. Increased serum interleukin 22 levels in patients with axial spondyloarthritis. Expert Rev. Clin. Immunol. 19, 123–129 (2023).
Yang, W. et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11, 4457 (2020).
van Praet, L. et al. Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann. Rheum. Dis. 73, 1186–1189 (2014).
Karow, F. et al. Monocyte transcriptomes from patients with axial spondyloarthritis reveal dysregulated monocytopoiesis and a distinct inflammatory imprint. Arthritis Res. Ther. 23, 246 (2021).
Yáñez, A. et al. Granulocyte-monocyte progenitors and monocyte-dendritic cell progenitors independently produce functionally distinct monocytes. Immunity 47, 890–902.e4 (2017).
Regan-Komito, D. et al. GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis. Nat. Commun. 11, 155 (2020).
Shi, H. et al. GM-CSF primes proinflammatory monocyte responses in ankylosing spondylitis. Front. Immunol. 11, 1520 (2020).
Mauro, D. & Ciccia, F. Gut dysbiosis in spondyloarthritis: cause or effect? Best Pract. Res. Clin. Rheumatol. 33, 101493 (2020).
Chavakis, T., Mitroulis, I. & Hajishengallis, G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 20, 802–811 (2019).
Ciccia, F. et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann. Rheum. Dis. 76, 1123–1132 (2017).
Guggino, G. et al. Inflammasome activation in ankylosing spondylitis is associated to gut dysbiosis. Arthritis Rheumatol. 73, 1189–1199 (2021).
Furesi, G. et al. Rodent models of spondyloarthritis have decreased white and bone marrow adipose tissue depots. Front. Immunol. 12, 665208 (2021).
Laurenti, E. & Göttgens, B. From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426 (2018).
Hurwitz, S. N., Jung, S. K. & Kurre, P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia 34, 3136–3148 (2020).
Gao, X., Xu, C., Asada, N. & Frenette, P. S. The hematopoietic stem cell niche: from embryo to adult. Development 145, dev139691 (2018).
Perry, J. M. & Li, L. in Cellular Programming and Reprogramming. Methods in Molecular Biology Vol. 636 (ed. Ding, S.) 45–54 (Humana, 2010).
Eaves, C. J. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 125, 2605–2613 (2015).
Vandoorne, K. et al. Imaging the vascular bone marrow niche during inflammatory stress. Circ. Res. 123, 415–427 (2018).
Olmsted-Davis, E. A. et al. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc. Natl Acad. Sci. USA 100, 15877–15882 (2003).
Ria, R. et al. Endothelial differentiation of hematopoietic stem and progenitor cells from patients with multiple myeloma. Clin. Cancer Res. 14, 1678–1685 (2008).
Bernardo, M. E. & Fibbe, W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 (2013).
He, X. et al. TLR4 activation promotes bone marrow MSC proliferation and osteogenic differentiation via Wnt3a and Wnt5a signaling. PLoS ONE 11, e0149876 (2016).
Pevsner-Fischer, M. et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109, 1422–1432 (2007).
Jung, Y. et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cell 26, 2042–2051 (2008).
Durig, J. et al. Intercellular communication between bone marrow stromal cells and CD34+ haematopoietic progenitor cells is mediated by connexin 43-type gap junctions. Br. J. Haematol. 111, 416–425 (2000).
Li, Y. et al. Whole genome expression profiling and signal pathway screening of MSCs in ankylosing spondylitis. Stem Cell Int. 2014, 913050 (2014).
Li, J. et al. Elevated TRAF4 expression impaired LPS-induced autophagy in mesenchymal stem cells from ankylosing spondylitis patients. Exp. Mol. Med. 49, e343 (2017).
Li, Y. et al. A study of the immunoregulatory function of TLR3 and TLR4 on mesenchymal stem cells in ankylosing spondylitis. Stem Cell Dev. 28, 1398–1412 (2019).
Li, Y.-X. et al. Integrative analysis of long non-coding RNA and messenger RNA expression in toll-like receptor 4-primed mesenchymal stem cells of ankylosing spondylitis. Ann. Transl. Med. 9, 1563–1563 (2021).
Yu, W. et al. SNP-adjacent super enhancer network mediates enhanced osteogenic differentiation of MSCs in ankylosing spondylitis. Hum. Mol. Genet. 30, 277–293 (2021).
Xie, Z. et al. Differential expression profiles of long noncoding RNA and mRNA of osteogenically differentiated mesenchymal stem cells in ankylosing spondylitis. J. Rheumatol. 43, 1523–1531 (2016).
Akiyama, K. et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10, 544–555 (2012).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008).
Li, D. et al. All-trans retinoic acid improves the effects of bone marrow-derived mesenchymal stem cells on the treatment of ankylosing spondylitis: an in vitro study. Stem Cell Int. 2015, 484528 (2015).
Ma, M. et al. SMAD-specific E3 ubiquitin ligase 2 promotes angiogenesis by facilitating PTX3 degradation in MSCs from patients with ankylosing spondylitis. Stem Cell 39, 581–599 (2021).
Liu, L., Yuan, Y., Zhang, S., Xu, J. & Zou, J. Osteoimmunological insights into the pathogenesis of ankylosing spondylitis. J. Cell. Physiol. 236, 6090–6100 (2021).
Zheng, G. et al. Enhanced osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis: a study based on a three-dimensional biomimetic environment. Cell Death Dis. 10, 350 (2019).
Xie, Z. et al. Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 68, 430–440 (2016).
Yang, Y. & Dai, M. Expression of PADI4 in patients with ankylosing spondylitis and its role in mediating the effects of TNF-α on the proliferation and osteogenic differentiation of human mesenchymal stem cells. Int. J. Mol. Med. 36, 565–570 (2015).
Liu, C.-H. et al. HLA-B27-mediated activation of TNAP phosphatase promotes pathogenic syndesmophyte formation in ankylosing spondylitis. J. Clin. Invest. 129, 5357–5373 (2019).
Dangoria, N. S. et al. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 277, 23459–23468 (2002).
Tran, T. M. et al. HLA-B27 in transgenic rats forms disulfide-linked heavy chain oligomers and multimers that bind to the chaperone BiP. J. Immunol. 172, 5110–5119 (2004).
Turner, M. J., DeLay, M. L., Bai, S., Klenk, E. & Colbert, R. A. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 56, 215–223 (2007).
Liu, C. et al. Transfer of microRNA-22-3p by M2 macrophage-derived extracellular vesicles facilitates the development of ankylosing spondylitis through the PER2-mediated Wnt/β-catenin axis. Cell Death Discov. 8, 269 (2022).
Liu, W. et al. Abnormal inhibition of osteoclastogenesis by mesenchymal stem cells through the miR-4284/CXCL5 axis in ankylosing spondylitis. Cell Death Dis. 10, 188 (2019).
Xie, Z. et al. TNF-α-mediated m6A modification of ELMO1 triggers directional migration of mesenchymal stem cell in ankylosing spondylitis. Nat. Commun. 12, 5373 (2021).
Osta, B., Lavocat, F., Eljaafari, A. & Miossec, P. Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front. Immunol. 5, 425 (2014).
Okamoto, K. & Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 9, a031245 (2019).
Jo, S. et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 20, 115 (2018).
Russell, T. et al. IL-17A and TNF modulate normal human spinal entheseal bone and soft tissue mesenchymal stem cell osteogenesis, adipogenesis, and stromal function. Cells 10, 341 (2021).
Papagoras, C. et al. IL‐17A expressed on neutrophil extracellular traps promotes mesenchymal stem cell differentiation toward bone‐forming cells in ankylosing spondylitis. Eur. J. Immunol. 51, 930–942 (2021).
McGonagle, D., Thomas, R. C. & Schett, G. Spondyloarthritis: may the force be with you? Ann. Rheum. Dis. 73, 321–323 (2014).
Tinazzi, I. et al. ‘Deep Koebner’ phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann. Rheum. Dis. 77, 922–925 (2018).
François, R. J., Gardner, D. L., Degrave, E. J. & Bywaters, E. G. L. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis: systematic study of specimens from patients and control subjects. Arthritis Rheum. 43, 2011–2024 (2000).
Bywaters, E. G. L. in Spondylarthropathies (ed. Calin, A.) 43–68 (Grune & Stratton, 1984).
Raman, N., Imran, S. A. M., Ahmad Amin Noordin, K. B., Zaman, W. S. W. K. & Nordin, F. Mechanotransduction in mesenchymal stem cells (MSCs) differentiation: a review. Int. J. Mol. Sci. 23, 4580 (2022).
Liu, Y.-S. & Lee, O. K. In search of the pivot point of mechanotransduction: mechanosensing of stem cells. Cell Transpl. 23, 1–11 (2014).
Hao, J. et al. Mechanobiology of mesenchymal stem cells: perspective into mechanical induction of MSC fate. Acta Biomater. 20, 1–9 (2015).
Pittenger, M. F. et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22 (2019).
Cambré, I. et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 9, 4613 (2018).
Yeremenko, N. et al. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. 65, 174–185 (2013).
Marinova-Mutafchieva, L., Williams, R. O., Funa, K., Maini, R. N. & Zvaifler, N. J. Inflammation is preceded by tumor necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum. 46, 507–513 (2002).
Wu, D., Schaffler, M. B., Weinbaum, S. & Spray, D. C. Matrix-dependent adhesion mediates network responses to physiological stimulation of the osteocyte cell process. Proc. Natl Acad. Sci. USA 110, 12096–12101 (2013).
Si, J. et al. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. 26, e919159 (2020).
Dai, B. et al. Macrophages in epididymal adipose tissue secrete osteopontin to regulate bone homeostasis. Nat. Commun. 13, 427 (2022).
Ishijima, M. et al. Osteopontin is required for mechanical stress-dependent signals to bone marrow cells. J. Endocrinol. 193, 235–243 (2007).
Choi, S. T. et al. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology 47, 1775–1779 (2008).
Yager, N. et al. Ex vivo mass cytometry analysis reveals a profound myeloid proinflammatory signature in psoriatic arthritis synovial fluid. Ann. Rheum. Dis. 80, 1559–1567 (2021).
Kaaij, M. H. et al. Anti-IL-17A treatment reduces serum inflammatory, angiogenic and tissue remodeling biomarkers accompanied by less synovial high endothelial venules in peripheral spondyloarthritis. Sci. Rep. 10, 21094 (2020).
Zhu, C., Chen, W., Lou, J., Rittase, W. & Li, K. Mechanosensing through immunoreceptors. Nat. Immunol. 20, 1269–1278 (2019).
McWhorter, F. Y., Davis, C. T. & Liu, W. F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 72, 1303–1316 (2015).
Symons, R. A. et al. Targeting the IL-6–Yap–Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann. Rheum. Dis. 81, 214–224 (2022).
Ekpenyong, A. E. et al. Mechanical deformation induces depolarization of neutrophils. Sci. Adv. 3, e1602536 (2017).
Kang, J. H. et al. Biomechanical forces enhance directed migration and activation of bone marrow-derived dendritic cells. Sci. Rep. 11, 12106 (2021).
Solis, A. G. et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 573, 69–74 (2019).
Jaumard, N. V., Welch, W. C. & Winkelstein, B. A. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J. Biomech. Eng. 133, 71010 (2011).
Yang, K. H. & King, A. I. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine 9, 557–565 (1984).
Kiapour, A. et al. Biomechanics of the sacroiliac joint: anatomy, function, biomechanics, sexual dimorphism, and causes of pain. Int. J. Spine Surg. 14, S3–S13 (2020).
Rosine, N. & Miceli-Richard, C. Innate cells: the alternative source of IL-17 in axial and peripheral spondyloarthritis? Front. Immunol. 11, 553742 (2021).
Maerz, T. et al. Acute mobilization and migration of bone marrow-derived stem cells following anterior cruciate ligament rupture. Osteoarthr. Cartil. 25, 1335–1344 (2017).
Chang, N.-H., Inman, R. D., Dick, J. E. & Wither, J. E. Bone marrow-derived human hematopoietic stem cells engraft NOD/SCID mice and traffic appropriately to an inflammatory stimulus in the joint. J. Rheumatol. 37, 496–502 (2010).
Schett, G. & Firestein, G. S. Mr Outside and Mr Inside: classic and alternative views on the pathogenesis of rheumatoid arthritis. Ann. Rheum. Dis. 69, 787–789 (2010).
Binks, D. A. et al. Role of vascular channels as a novel mechanism for subchondral bone damage at cruciate ligament entheses in osteoarthritis and inflammatory arthritis. Ann. Rheum. Dis. 74, 196–203 (2015).
de Winter, J. et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the Assessment of SpondyloArthritis international Society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 70, 1042–1048 (2018).
Author information
Authors and Affiliations
Contributions
D.M., S.G., G.S., W.P.M. and F.C. researched data and wrote the article. All authors made a substantial contribution to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks R. Colbert, R. Ramonda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mauro, D., Gandolfo, S., Tirri, E. et al. The bone marrow side of axial spondyloarthritis. Nat Rev Rheumatol 19, 519–532 (2023). https://doi.org/10.1038/s41584-023-00986-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-00986-6
This article is cited by
-
Effects of disease-modifying anti-rheumatic drugs on sacroiliac MRI score in axial spondyloarthritis: a systematic review and meta-analysis
Clinical Rheumatology (2024)
-
Is sarcopenia a real concern in ankylosing spondylitis? A systematic literature review
European Geriatric Medicine (2024)
-
The Role of Early Treatment in the Management of Axial Spondyloarthritis: Challenges and Opportunities
Rheumatology and Therapy (2024)
-
Predominant ligament-centric soft-tissue involvement differentiates axial psoriatic arthritis from ankylosing spondylitis
Nature Reviews Rheumatology (2023)