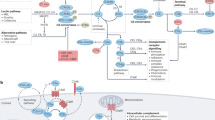
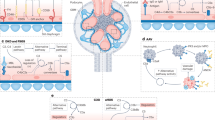
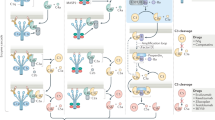
Abstract
The complement system was described over 100 years ago, and it is well established that activation of this pathway accompanies the great majority of autoimmune and inflammatory diseases. In addition, over three decades of work in murine models of human disease have nearly universally demonstrated that complement activation is upstream of tissue injury and the engagement of pro-inflammatory mechanisms such as the elaboration of cytokines and chemokines, as well as myeloid cell recruitment and activation. With that background, and taking advantage of advances in the development of biologic and small-molecule therapeutics, the creation and clinical evaluation of complement therapeutics is now rapidly expanding. This article reviews the current state of the complement therapeutics field, with a focus on their use in diseases cared for or consulted upon by rheumatologists. Included is an overview of the activation mechanisms and components of the system, in addition to the mechanisms by which the complement system interacts with other immune system constituents. The various therapeutic approaches to modulating the system in rheumatic and autoimmune diseases are reviewed. To understand how best to clinically assess the complement system, methods of its evaluation are described. Finally, next-generation therapeutic and diagnostic advances that can be envisioned for the future are discussed.
Key points
-
Analyses of both tissue and circulating biomarkers indicate that the complement pathway is activated in the majority of rheumatological and autoimmune diseases.
-
A complement inhibitor targeting interaction of the anaphylatoxin C5a with its receptor is effective and approved for the treatment of ANCA-associated vasculitis.
-
A separate inhibitor of C5 activation is also effective and approved for use in atypical haemolytic uraemic syndrome (aHUS), a thrombotic microangiopathy disorder, as well as neuromyelitis optica spectrum disorder.
-
Complement system biomarkers assessed using multiple formats can provide diagnostic as well as prognostic information in diseases for which there are approved complement therapeutics, as well as in certain other clinical settings.
-
Murine models of human rheumatic and autoimmune diseases, including ANCA-associated vasculitis, aHUS, thrombotic microangiopathy and lupus nephritis, demonstrate that complement activation is upstream of tissue injury and the generation of other effector mechanisms.
-
Polymorphic variation and mutations in complement genes are associated with the risks of development of several rheumatic and autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2014).
Holers, V. M. Complement and its receptors: new insights into human disease. Ann. Rev. Immunol. 32, 433–459 (2014).
Ricklin, D., Mastellos, D. C., Reis, E. S. & Lambris, J. D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14, 26–47 (2018).
Liszewski, M. K., Java, A., Schramm, E. C. & Atkinson, J. P. Complement dysregulation and disease: insights from contemporary genetics. Annu. Rev. Pathol. Mech. Dis. 12, 25–52 (2017).
Ricklin, D. & Lambris, J. D. Progress and trends in complement therapeutics. Adv. Exp. Med. Biol. 735, 1–22 (2013).
Jayne, D. R. W., Merkel, P. S., Schall, T. J., Bekker, P. & Group, A. S. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
Nürnberger, J. et al. Eculizumab for atypical hemolytic-uremic syndrome. N. Engl. J. Med. 360, 542–544 (2009).
Kim, M. Y. et al. Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann. Rheum. Dis. 77, 549–555 (2018).
Li, N. L., Birmingham, D. J. & Rovin, B. H. Expanding the role of complement therapies: the case for lupus nephritis. J. Clin. Med. 10, 626 (2021).
Holers, V. M., Kinoshita, T. & Molina, H. Evolution of mouse and human complement of C3 binding proteins: divergence of form but conservation of function. Immunol. Today 13, 231–236 (1992).
Banda, N. & Holers, V. M. Complement in the initiation and evolution of rheumatoid arthritis. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01057 (2018).
Walport, M. J. Complement: first of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Walport, M. J. Complement: second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001).
Bohlson, S. S., Garred, P., Kemper, C. & Tenner, A. J. Complement nomenclature — deconvoluted. Front. Immunol. https://doi.org/10.3389/fimmu.2019.01308 (2019).
Li, K., Sacks, S. H. & Zhou, W. The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol. Immunol. 44, 3866–3874 (2007).
Lachmann, P. J. & Hughes-Jones, N. C. Initiation of complement activation. Springer Semin. Immunopathol. 7, 143–162 (1984).
Garred, P. et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol. Rev. 274, 74–97 (2016).
Rademacher, T. W., Williams, P. & Dwek, R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl Acad. Sci. USA 91, 6123–6127 (1994).
Haddad, G. et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J. Clin. Invest. 131, e140453 (2021).
Takahashi, M. et al. Essential role of Mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J. Exp. Med. 207, 29–37 (2010).
Nilsson, B. & Ekdahl, K. N. The tick-over theory revisited: Is C3 a contact-activated protein? Immunobiology 217, 1106–1110 (2012).
Lachmann, P. J. The amplification loop of the complement pathways. Adv. Immunol. 104, 115–149 (2009).
Holers, V. M. Contributions of animal models to mechanistic understandings of antibody-dependent disease and roles of the amplification loop. Immunol. Rev. 313, 181–193 (2022).
Ricklin, D., Reis, E. S., Mastellos, D. C., Gros, P. & Lambris, J. D. Complement component C3 — the “Swiss Army Knife” of innate immunity and host defense. Immunol. Rev. 274, 33–58 (2016).
Xie, C. B., Jane-Wit, D. & Pober, J. S. Complement membrane attack complex new roles, mechanisms of action, and therapeutic targets. Am. J. Pathol. 190, 1138–1150 (2020).
Cicardi, M. & Johnston, D. T. Hereditary and acquired complement component 1 esterase inhibitor deficiency: a review for the hematologist. Acta Haematol. 127, 208–220 (2012).
Zipfel, P. F. et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 67, 21–30 (2015).
Parente, R., Clark, S. J., Inforzato, A. & Day, A. J. Complement factor H in host defense and immune evasion. Cell. Mol. Life Sci. 74, 1605–1624 (2017).
Ermert, D. & Blom, A. M. C4b-binding protein: the good, the bad and the deadly. Novel functions of an old friend. Immunol. Lett. 169, 82–92 (2016).
Liszewski, M. K., Farries, T. C., Lublin, D. M., Rooney, I. A. & Atkinson, J. P. Control of the complement system. Adv. Immunol. 61, 201–283 (1996).
Kim, D. D. & Song, W.-C. Membrane complement regulatory proteins. Clin. Immunol. 118, 127–136 (2006).
Matthews, K. W., Mueller-Ortiz, S. L. & Wetsel, R. A. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol. Immunol. 40, 785–793 (2004).
Gialeli, C., Gungor, B. & Blom, A. M. Novel potential inhibitors of complement system and their roles in complement regulation and beyond. Mol. Immunol. 102, 73–83 (2018).
de Cordoba, S. R., Tortajada, A., Harris, C. L. & Morgan, B. P. Complement dysregulation and disease: from genes and proteins to diagnostics and drugs. Immunobiology 217, 1034–1046 (2012).
Medjeral‐Thomas, N. & Pickering, M. C. The complement factor H‐related proteins. Immunol. Rev. 274, 191–201 (2016).
Jozsi, M. & Meri, S. Factor H-related proteins. Methods Mol. Biol. 1100, 225–236 (2014).
Poppelaars, F. et al. A family affair: addressing the challenges of Factor H and the related proteins. Front. Immunol. 12, 660194 (2021).
Banerjeea, P. et al. Evaluating the clinical utility of measuring levels of factor H and the related proteins. Mol. Immunol. 151, 166–182 (2022).
Carroll, M. C. & Isenman, D. E. Regulation of humoral immunity by complement. Immunity 37, 199–207 (2012).
Sayegh, E. T., Bloch, O. & Parsa, A. T. Complement anaphylatoxins as immune regulators in cancer. Cancer Med. 3, 747–758 (2014).
Kavai, M. Immune complex clearance by complement receptor type 1 in SLE. Autoimmun. Rev. 8, 160–164 (2008).
Carroll, M. C. The role of complement in B cell activation and tolerance. Adv. Immunol. 74, 61–88 (2000).
van Lookeren Campagne, M., Wiesmann, C. & Brown, E. J. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 9, 2095–2102 (2007).
Carroll, M. C. & Holers, V. M. Innate autoimmunity. Adv. Immunol. 86, 137–157 (2005).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Helmy, K. Y. et al. CRIg: a macrophage complement receptor required for phagocytosis and circulating pathogens. Cell 124, 915–927 (2006).
Wetsel, R. A. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr. Opin. Immunol. 7, 48–53 (1995).
Pandey, S., Maharana, J., Li, X. X., Woodruff, T. M. & Shukla, A. K. Emerging insights into the structure and function of complement C5a receptors. Trends Biochem. Sci. 45, 693–705 (2020).
Karsten, C. M. & Kohl, J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology 217, 1067–1079 (2012).
Humbles, A. A. et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature 406, 998–1001 (2000).
Gao, S. & Cui, Z. & Zhao, M.-h. The complement C3a and C3a receptor pathway in kidney diseases. Front. Immunol. https://doi.org/10.3389/fimmu.2020.01875 (2020).
Kunz, N. & Kemper, C. Complement has brains — do intracellular complement and immunometabolism cooperate in tissue homeostasis and behavior? Front. Immunol. https://doi.org/10.3389/fimmu.2021.629986 (2021).
King, B. C. & Blom, A. M. Intracellular complement: evidence, definitions, controversies, and solutions. Immunol. Rev. 313, 104–119 (2022).
Heurich, M. et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl Acad. Sci. USA 108, 8761–8766 (2011).
Kamitak, N. et al. Complement genes contribute sex-biased vulnerability in diverse illnesses. Nature 582, 577–581 (2020).
Rother, R. P., Rollins, S. A., Mojcik, C. F., Brodsky, R. A. & Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 25, 1256–1264 (2007).
Nester, C. M. & Brophy, P. D. Eculizumab in the treatment of atypical haemolytic uraemic syndrome and other complement-mediated renal diseases. Curr. Opin. Pediatr. 25, 225–231 (2012).
Pittock, S. J. et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 381, 614–625 (2019).
Howard, J. F. Jr et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 16, 976–986 (2017).
Varga, L. & Farkas, H. rhC1INH: a new drug for the treatment of attacks in hereditary angioedema caused by C1-inhibitor deficiency. Expert. Rev. Clin. Immunol. 7, 143–153 (2011).
Mastellos, D. C. et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Invest. 45, 423–440 (2015).
Hillmen, P. et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 384, 1028–1037 (2021).
Berentsen, S., Barcellini, W., D’Sa, S. & Jilma, B. Sutimlimab for treatment of cold agglutinin disease: why, how and for whom? Immunotherapy 14, 1191–1204 (2022).
Holers, V. M. et al. New therapeutic and diagnostic opportunities for injured tissue-specific targeting of complement inhibitors and imaging modalities. Semin. Immunol. 28, 260–267 (2016).
Botto, M. et al. Complement in human diseases: lessons from complement deficiencies. Mol. Immunol. 46, 2774–2783 (2009).
Kitching, A. R. et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 6, 71 (2020).
Jain, R., Jawa, P., Derebail, V. K. & Falk, R. J. Treatment updates in antineutrophil cytoplasmic autoantibodies (ANCA) vasculitis. Kidney360 2, 763–770 (2021).
Heijl, C., Mohammad, A. J., Westman, K. & Höglund, P. Long-term patient survival in a Swedish population-based cohort of patients with ANCA-associated vasculitis. RMD Open https://doi.org/10.1136/rmdopen-2017-000435 (2017).
Falk, R. J., Terrell, R. S., Charles, L. A. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl Acad. Sci. USA 87, 4115–4119 (1990).
O’Sullivan, K. M. & Holdsworth, S. R. Neutrophil extracellular traps: a potential therapeutic target in MPO-ANCA associated vasculitis? Front. Immunol. https://doi.org/10.3389/fimmu.2021.635188 (2021).
Schreiber, A. et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl Acad. Sci. USA 114, E9618–E9625 (2017).
Stone, J. H. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232 (2010).
Wu, E. Y. et al. Measuring circulating complement activation products in myeloperoxidase- and proteinase 3-antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 71, 1894–1903 (2019).
Kallenberg, C. G. M. & Heeringa, P. Complement is crucial in the pathogenesis of ANCA-associated vasculitis. Kidney Int. 83, 16–18 (2012).
Johansson, L. et al. Complement activation prior to symptom onset in myeloperoxidase ANCA-associated vasculitis but not proteinase 3 ANCA associated vasculitis — a Swedish biobank study. Scand. J. Rheum. 51, 214–219 (2022).
Gou, S. J., Yuan, J., Wang, C., Zhao, M.-H. & Chen, M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin. J. Am. Soc. Nephrol. 8, 1884–1891 (2013).
Oba, R. et al. Long-term renal survival in antineutrophil cytoplasmic antibody-associated glomerulonephritis with complement C3 deposition. Kidney Int. Rep. 6, 2661–2670 (2021).
Shochet, L., Holdsworth, S. & Kitching, A. R. Animal models of ANCA associated vasculitis. Front. Immunol. https://doi.org/10.3389/fimmu.2020.00525 (2020).
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J. & Jennette, J. C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007).
Xiao, H. et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J. Am. Soc. Nephrol. 25, 225–231 (2014).
Miao, D., Li, D.-Y., Chen, M. & Zhao, M.-H. Platelets are activated in ANCA-associated vasculitis via thrombin-PARs pathway and can activate the alternative complement pathway. Arthritis Res. Ther. 19, 252 (2017).
Noris, M. & Remuzzi, G. Hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 1035–1050 (2005).
Noris, M. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 5, 1844–1859 (2010).
Perez-Caballero, D. et al. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 68, 478–484 (2001).
Neumann, H. P. et al. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J. Med. Genet. 40, 676–681 (2003).
Caprioli, J. et al. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum. Mol. Genet. 12, 3385–3395 (2003).
Richards, A. et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA 100, 12966–12971 (2003).
Noris, M. et al. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet 362, 1542–1547 (2003).
Fremeaux-Bacchi, V. et al. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J. Med. Genet. 41, e84 (2004).
Dragon-Durey, M. A. et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 555–563 (2005).
Dragon-Durey, M. A. et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J. Am. Soc. Nephrol. 21, 2180–2187 (2010).
Arjona, E., Huerta, A., Goicoechea de Jorge, E. & Rodrıguez de Cordoba, S. Familial risk of developing atypical hemolytic-uremic syndrome. Blood 136, 1558–1561 (2020).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
El-Husseini, A. et al. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizumab. Am. J. Kidney Dis. 65, 127–130 (2014).
Devresse, A. et al. Complement activation and effect of eculizumab in scleroderma renal crisis. Medicine 95, e4459 (2016).
Asif, A., Nayer, A. & Haas, C. S. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J. Nephrol. 30, 347–362 (2017).
Wingerchuk, D. M. & Lucchinetti, C. F. Neuromyelitis optica spectrum disorder. N. Engl. J. Med. 387, 631–639 (2022).
Bennett, J. L. & Owens, G. P. Neuromyelitis optica: deciphering a complex immune-mediated astrocytopathy. J. Neuroophthalmol. 37, 291–299 (2017).
Hakobyan, S. et al. Plasma complement biomarkers distinguish multiple sclerosis and neuromyelitis optica spectrum disorder. Mult. Scler. 23, 946–955 (2017).
Duan, T. & Verkman, A. S. Experimental animal models of aquaporin-4-IgG-seropositive neuromyelitis optica spectrum disorders: progress and shortcomings. Brain Pathol. 30, 13–25 (2020).
Pittock, S. J. et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 12, 554–562 (2013).
Fanouriakis, A., Tziolos, N., Bertsias, G. & Boumpas, D. T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 80, 14–25 (2021).
Zhao, J. et al. Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. https://doi.org/10.1371/journal.pgen.1002079 (2011).
Wangm, F. M., Yu, F., Tan, Y., Song, D. & Zhao, M. H. Serum complement factor H is associated with clinical and pathological activities of patients with lupus nephritis. Rheumatology 51, 2269–2277 (2012).
Kim, A. H. J. et al. Association of blood concentrations of complement split product iC3b and serum C3 with systemic lupus erythematosus disease activity. Arthritis Rheum. 71, 420–430 (2019).
Martin, M. et al. Plasma C4d correlates with C4d deposition in kidneys and with treatment response in lupus nephritis patients. Front. Immunol. https://doi.org/10.3389/fimmu.2020.582737 (2020).
Buyon, J. P., Tamerius, J., Ordorica, S., Young, B. & Abramson, S. B. Activation of the alternative complement pathway accompanies disease flares in systemic lupus erythematosus during pregnancy. Arthritis Rheum. 35, 55–61 (1992).
Parikh, S. V. et al. Molecular imaging of the kidney in lupus nephritis to characterize response to treatment. Transl. Res. 182, 1–13 (2017).
Sato, N. et al. Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus 20, 1378–1386 (2011).
Hill, G. S. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 59, 304–316 (2001).
Wang, Y. et al. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc. Natl Acad. Sci. USA 93, 8563–8568 (1996).
Watanabe, H. et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J. Immunol. 164, 786–794 (2000).
Elliott, M. K. et al. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int. 65, 129–138 (2004).
Bao, L. et al. Transgenic expression of a soluble complement inhibitor protects against renal disease and promotes survival in MRL/lpr mice. J. Immunol. 168, 3601–3607 (2002).
Lieberman, L. A. et al. Complement receptor of the immunoglobulin superfamily reduces murine lupus nephritis and cutaneous disease. Clin. Immunol. 160, 286–291 (2015).
Bao, L. et al. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur. J. Immunol. 35, 2496–2506 (2005).
Sekine, H. et al. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J. Immunol. 166, 6444–6451 (2001).
Pickering, M. C. et al. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology 54, 2286–2288 (2015).
Garcia, D. & Erkan, D. Diagnosis and management of the antiphospholipid syndrome. N. Engl. J. Med. 378, 2010–2021 (2018).
Chaturvedi, S., Braunstein, E. M. & Brodsky, R. A. Antiphospholipid syndrome: complement activation, complement gene mutations, and therapeutic implications. J. Thromb. Haemost. 19, 607–616 (2021).
Chaturvedi, S., Brodsky, R. A. & McCrae, K. R. Complement in the pathophysiology of the antiphospholipid syndrome. Front. Immunol. 10, 449 (2019).
Breen, K. A. et al. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb. Haemost. 107, 423–429 (2012).
Chaturvedi, S. et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood 135, 239–251 (2020).
Strakham, M. et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep. Hematol. https://doi.org/10.1155/2014/704371 (2014).
Holers, V. M. et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 195, 211–220 (2002).
Pierangelli, S. et al. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 52, 2120–2124 (2005).
Girardi, G. et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 112, 1644–1654 (2003).
Girardi, G., Redecha, P. & Salmon, J. E. Heparin prevents anti-phospholipid antibody-mediated fetal loss by inhibiting complement activation. Nat. Med. 10, 1222–1226 (2004).
Girardi, G., Yarilin, D., Thurman, J. M., Holers, V. M. & Salmon, J. E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 203, 2165–2175 (2006).
Wang, Q. et al. Identification of a role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Lahoria, R., Selcen, D. & Engel, A. G. Microvascular alterations and the role of complement in dermatomyositis. Brain 139, 1891–1903 (2016).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Humby, F. et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann. Rheum. Dis. 78, 761–772 (2019).
Lewis, M. J. et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 28, 2455–2470 (2019).
Linton, S. M. & Morgan, B. P. Complement activation and inhibition in experimental models of arthritis. Mol. Immunol. 36, 905–914 (1999).
Monach, P. A., Benoist, C. & Mathis, D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv. Immunol. 82, 217–248 (2004).
Neumann, E. et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 46, 934–945 (2002).
Mojcik, C. F. et al. Results of a phase 2b study of the humanized anti-C5 antibody eculizumab in patients with rheumatoid arthritis [abstract]. Ann. Rheum. Dis. 63, 301 (2004).
Banda, N. K. et al. Analysis of complement gene expression, clinical associations, and biodistribution of complement proteins in the synovium of early rheumatoid arthritis patients reveals unique pathophysiologic features. J. Immunol. 208, 2482–2496 (2022).
Hitchon, C. A. & El-Gabalawy, H. S. The synovium in rheumatoid arthritis. Open Rheumatol. 5, 107–114 (2011).
Paoliello-Paschoalato, A. B. et al. Activation of complement alternative pathway in rheumatoid arthritis: implications in peripheral neutrophils functions. Open. Autoimmun. J. 3, 1–9 (2014).
Dalakas, M. C. Complement in autoimmune inflammatory myopathies, the role of myositis-associated antibodies, COVID-19 associations, and muscle amyloid deposits. Expert. Rev. Clin. Immunol. https://doi.org/10.1080/1744666X.2022.2054803 (2022).
Prohászka, Z., Nilsson, B., Frazer-Abel, A. & Kirschfink, M. Complement analysis 2016: clinical indications, laboratory diagnostics and quality control. Immunobiology 221, 1247–1258 (2016).
Ohtani, K. Complement-related proteins and their measurements: the current status of clinical investigation. Nephron 144, 7–12 (2020).
Mollnes, T. E. et al. Complement analysis in the 21st century. Mol. Immunol. 44, 3838–3849 (2007).
Palarasah, Y. et al. Novel assays to assess the functional capacity of the classical, the alternative and the lectin pathways of the complement system. Clin. Exp. Immunol. 164, 388–395 (2011).
Harder, M. J. et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood 129, 970–980 (2017).
Bu, F. et al. Soluble c5b-9 as a biomarker for complement activation in atypical hemolytic uremic syndrome. Am. J. Kidney Dis. 65, 968–969 (2015).
Muff-Luett, M. & Nester, C. M. The genetics of ultra-rare renal disease. J. Pediatr. Genet. 5, 33–42 (2016).
van Lookeren Campagne, M., Strauss, E. C. & Yaspan, B. L. Age-related macular degeneration: complement in action. Immunobiology 221, 733–739 (2016).
Barnum, S. R., Bubeck, D. & Schein, T. N. Soluble membrane attack complex: biochemistry and immunobiology. Front. Immunol. https://doi.org/10.3389/fimmu.2020.585108 (2020).
Zwarthoff, S. A. et al. Functional characterization of alternative and classical pathway C3/C5 convertase activity and inhibition using purified models. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01691 (2018).
Trouw, L. A. et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J. Clin. Invest. 114, 679–688 (2004).
Ramsey-Goldman, R., Li, J., Dervieux, T. & Alexander, R. V. Cell-bound complement activation products in SLE. Lupus Sci. Med. 4, e000236 (2017).
Ramsey-Goldman, R. et al. Complement activation in patients with probable Systemic Lupus Erythematosus and ability to predict progression to American College of Rheumatology — classified systemic lupus erythematosus. Arthritis Rheumatol. 72, 78–88 (2020).
Thurman, J. M. et al. Detection of complement activation using monoclonal antibodies against C3d. J. Clin. Invest. 123, 2218–2230 (2013).
Willems, E. et al. Quantitative multiplex profiling of the complement system to diagnose complement-mediated diseases. Clin. Transl. Immunol. 9, e1225 (2020).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009).
Smith-Jackson, K. et al. Hyperfunctional complement C3 promotes C5-dependent atypical hemolytic uremic syndrome in mice. J. Clin. Invest. 129, 1061–1075 (2019).
Sekine, H., Ruiz, P., Gilkeson, G. S. & Tomlinson, S. The dual role of complement in the progression of renal disease in NZB/W F1 mice and alternative pathway inhibition. Mol. Immunol. 49, 317–323 (2011).
Thurman, J. M. et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol. Immunol. 42, 87–97 (2005).
Acknowledgements
The author thanks his many colleagues with whom he has worked to understand the biological and clinical roles of the complement system in human disease. These individuals include J. Thurman, N. Banda, L. Kulik, F. Zhang, S. Tomlinson, R. Quigg, C. Atkinson, A. Lynch, J. Atkinson, B. Dixon and A. Frazer-Abel. The author also thanks colleagues from Taligen Therapeutics and Q32 Bio, who have worked to develop therapeutics designed to translate to patient care the discoveries made in academic research laboratories.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that he is a co-founder of Q32 Bio, a complement therapeutics company, and through those efforts has received sponsored research funding, consulting income and stock.
Peer review
Peer review information
Nature Reviews Rheumatology thanks A. Blom, L. Trouw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Holers, V.M. Complement therapeutics are coming of age in rheumatology. Nat Rev Rheumatol 19, 470–485 (2023). https://doi.org/10.1038/s41584-023-00981-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-00981-x