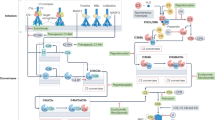
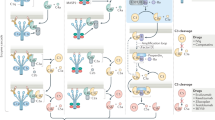
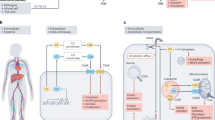
Abstract
Complement has long been considered a key innate immune effector system that mediates host defence and tissue homeostasis. Yet, growing evidence has illuminated a broader involvement of complement in fundamental biological processes extending far beyond its traditional realm in innate immunity. Complement engages in intricate crosstalk with multiple pattern-recognition and signalling pathways both in the extracellular and intracellular space. Besides modulating host–pathogen interactions, this crosstalk guides early developmental processes and distinct cell trajectories, shaping tissue immunometabolic and regenerative programmes in different physiological systems. This Review provides a guide to the system-wide functions of complement. It highlights illustrative paradigm shifts that have reshaped our understanding of complement pathobiology, drawing examples from evolution, development of the central nervous system, tissue regeneration and cancer immunity. Despite its tight spatiotemporal regulation, complement activation can be derailed, fuelling inflammatory tissue pathology. The pervasive contribution of complement to disease pathophysiology has inspired a resurgence of complement therapeutics with major clinical developments, some of which have challenged long-held dogmas. We thus highlight major therapeutic concepts and milestones in clinical complement intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hajishengallis, G., Reis, E. S., Mastellos, D. C., Ricklin, D. & Lambris, J. D. Novel mechanisms and functions of complement. Nat. Immunol. 18, 1288–1298 (2017).
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016).
Lambris, J. D., Ricklin, D. & Geisbrecht, B. V. Complement evasion by human pathogens. Nat. Rev. Microbiol. 6, 132–142 (2008).
Cerenius, L., Kawabata, S., Lee, B. L., Nonaka, M. & Söderhäll, K. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem. Sci. 35, 575–583 (2010).
Stevens, B. & Johnson, M. B. The complement cascade repurposed in the brain. Nat. Rev. Immunol. 21, 624–625 (2021).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I — molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Kolev, M., Friec, G. L. & Kemper, C. Complement — tapping into new sites and effector systems. Nat. Rev. Immunol. 14, 811–820 (2014).
Kjaer, T. R., Thiel, S. & Andersen, G. R. Toward a structure-based comprehension of the lectin pathway of complement. Mol. Immunol. 56, 413–422 (2013).
Garred, P. et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol. Rev. 274, 74–97 (2016).
Ricklin, D. Manipulating the mediator: modulation of the alternative complement pathway C3 convertase in health, disease and therapy. Immunobiology 217, 1057–1066 (2012).
Diebolder, C. A. et al. Complement is activated by IgG hexamers assembled at the cell surface. Science 343, 1260–1263 (2014). This study provided structural insights into the activation of the classical pathway by hexameric antigen–IgG complexes.
Ugurlar, D. et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359, 794–797 (2018).
Golay, J. & Taylor, R. P. The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies 9, 58 (2020).
Ricklin, D. & Lambris, J. D. Therapeutic control of complement activation at the level of the central component C3. Immunobiology 221, 740–746 (2015).
Ricklin, D., Reis, E. S., Mastellos, D. C., Gros, P. & Lambris, J. D. Complement component C3 — the ‘Swiss army knife’ of innate immunity and host defense. Immunol. Rev. 274, 33–58 (2016).
Coulthard, L. G. & Woodruff, T. M. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J. Immunol. 194, 3542–3548 (2015). This review revisits the role of anaphylatoxins and provides a conceptual framework for understanding their broad immunomodulatory functions.
Parsons, E. S. et al. Single-molecule kinetics of pore assembly by the membrane attack complex. Nat. Commun. 10, 2066 (2019).
Irmscher, S. et al. Kallikrein cleaves C3 and activates complement. J. Innate Immun. 10, 94–105 (2018).
Amara, U. et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185, 5628–5636 (2010).
Békássy, Z. D. et al. Aliskiren inhibits renin-mediated complement activation. Kidney Int. 94, 689–700 (2018).
Skendros, P. et al. Complement C3 inhibition in severe COVID-19 using compstatin AMY-101. Sci. Adv. 8, eabo2341 (2022). This study provides biological insights into C3 inhibition in severe COVID-19 and the first clinical proof of concept for extrinsic routes of C3 activation.
Liszewski, M. K. et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39, 1143–1157 (2013). This study provides evidence that complement activation may occur within various cells and may have broad physiological significance.
Puy, C. et al. Cross-talk between the complement pathway and the contact activation system of coagulation: activated factor XI neutralizes complement factor H. J. Immunol. 206, 1784–1792 (2021).
Wingrove, J. A. et al. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J. Biol. Chem. 267, 18902–18907 (1992).
Tosi, M. F., Zakem, H. & Berger, M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J. Clin. Invest. 86, 300–308 (1990).
Nilsson, P. H. et al. A conformational change of complement C5 is required for thrombin-mediated cleavage, revealed by a novel ex vivo human whole blood model preserving full thrombin activity. J. Immunol. 207, 1641–1651 (2021).
Dobó, J. et al. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci. Rep. 6, 31877 (2016). This study highlights the molecular basis of the tight interdependency of the lectin and alternative complement pathways.
Hayashi, M. et al. Cutting Edge: role of MASP-3 in the physiological activation of factor D of the alternative complement pathway. J. Immunol. 203, 1411–1416 (2019).
Selander, B. et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J. Clin. Invest. 116, 1425–1434 (2006).
Farrar, C. A. et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J. Clin. Invest. 126, 1911–1925 (2016).
Laich, A., Patel, H., Zarantonello, A., Sim, R. B. & Inal, J. M. C2 by-pass: cross-talk between the complement classical and alternative pathways. Immunobiology 227, 152225 (2022).
Mannes, M. et al. Complement inhibition at the level of C3 or C5: mechanistic reasons for ongoing terminal pathway activity. Blood 137, 443–455 (2021). This study challenges the traditional assembly of C5 convertases, demonstrating C3 bypass activation of C5 and conformational activation of C5, which can bypass therapeutic C5 inhibitors.
Carroll, M. C. & Isenman, D. E. Regulation of humoral immunity by complement. Immunity 37, 199–207 (2012).
Helmy, K. Y. et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927 (2006).
Sorbara, M. T. et al. Complement C3 drives autophagy-dependent restriction of cyto-invasive bacteria. Cell Host Microbe 23, 644–652.e5 (2018).
Lindorfer, M. A., Hahn, C. S., Foley, P. L. & Taylor, R. P. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol. Rev. 183, 10–24 (2001).
Thielen, A. J. F., Zeerleder, S. & Wouters, D. Consequences of dysregulated complement regulators on red blood cells. Blood Rev. 32, 280–288 (2018).
Torvell, M. et al. Genetic insights into the impact of complement in Alzheimer’s disease. Genes 12, 1990 (2021).
Reis, E. S., Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. New insights into the immune functions of complement. Nat. Rev. Immunol. 19, 503–516 (2019).
Fearon, D. T. & Carroll, M. C. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 18, 393–422 (2000).
Erdei, A. et al. New aspects in the regulation of human B cell functions by complement receptors CR1, CR2, CR3 and CR4. Immunol. Lett. 237, 42–57 (2021).
Kovács, K. G., Mácsik-Valent, B., Matkó, J., Bajtay, Z. & Erdei, A. Revisiting the coreceptor function of complement receptor type 2 (CR2, CD21); coengagement with the B-cell receptor inhibits the activation, proliferation, and antibody production of human B Cells. Front. Immunol. 12, 558 (2021).
Kulik, L. et al. Targeting the immune complex-bound complement C3d ligand as a novel therapy for lupus. J. Immunol. 203, 3136–3147 (2019).
Schriek, P. et al. Marginal zone B cells acquire dendritic cell functions by trogocytosis. Science 375, eabf7470 (2022). This study reveals a new role of C3 in antigen presentation via binding to peptide–MHC class II complexes on dendritic cells and CR2-dependent transfer of these complexes to marginal zone B cells.
Joly, E. & Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol. 4, 815–815 (2003).
Hawksworth, O. A., Li, X. X., Coulthard, L. G., Wolvetang, E. J. & Woodruff, T. M. New concepts on the therapeutic control of complement anaphylatoxin receptors. Mol. Immunol. 89, 36–43 (2017).
Klos, A. et al. The role of the anaphylatoxins in health and disease. Mol. Immunol. 46, 2753–2766 (2009).
Reis, E. S. et al. C5a receptor-dependent cell activation by physiological concentrations of desarginated C5a: insights from a novel label-free cellular assay. J. Immunol. 189, 4797–4805 (2012).
Wang, R., Lu, B., Gerard, C. & Gerard, N. P. C5L2, the second C5a anaphylatoxin receptor, suppresses LPS-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 55, 657–666 (2016).
Pandey, S., Maharana, J., Li, X. X., Woodruff, T. M. & Shukla, A. K. Emerging insights into the structure and function of complement C5a receptors. Trends Biochem. Sci. 45, 693–705 (2020).
Li, X. X., Clark, R. J. & Woodruff, T. M. C5aR2 activation broadly modulates the signaling and function of primary human macrophages. J. Immunol. 205, 1102–1112 (2020).
Reca, R. et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood 101, 3784–3793 (2003).
Wu, M. C. L. et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl Acad. Sci. USA 110, 9439–9444 (2013). This study challenges the dogma that C3a has only pro-inflammatory functions by providing in vivo evidence for an anti-inflammatory function in the context of ischaemia–reperfusion injury.
Bujko, K. et al. Signaling of the complement cleavage product anaphylatoxin C5a through C5aR (CD88) contributes to pharmacological hematopoietic stem cell mobilization. Stem Cell Rev. Rep. 13, 793–800 (2017).
Morgan, B. P., Boyd, C. & Bubeck, D. Molecular cell biology of complement membrane attack. Semin. Cell Dev. Biol. 72, 124–132 (2017).
Xie, C. B., Jane-Wit, D. & Pober, J. S. Complement membrane attack complex: new roles, mechanisms of action, and therapeutic targets. Am. J. Pathol. 190, 1138 (2020).
Liszewski, M. K. & Atkinson, J. P. Alternative pathway activation: ever ancient and ever new. Immunol. Rev. 313, 60–63 (2023).
Zipfel, P. F. & Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9, 729–740 (2009).
Józsi, M., Tortajada, A., Uzonyi, B., Goicoechea de Jorge, E. & Rodríguez de Córdoba, S. Factor H-related proteins determine complement-activating surfaces. Trends Immunol. 36, 374–384 (2015).
Makou, E., Herbert, A. P. & Barlow, P. N. Creating functional sophistication from simple protein building blocks, exemplified by factor H and the regulators of complement activation. Biochem. Soc. Trans. 43, 812–818 (2015).
Zipfel, P. F., Jokiranta, T. S., Hellwage, J., Koistinen, V. & Meri, S. The factor H protein family. Immunopharmacology 42, 53–60 (1999).
Forneris, F. et al. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J. 35, 1133–1149 (2016). This study provides a unifying structural framework for understanding complement regulation and microbial immune evasion at the level of the convertases.
Couves, E. C. et al. Structural basis for membrane attack complex inhibition by CD59. Nat. Commun. 14, 890 (2023).
Castellano, G. et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am. J. Pathol. 176, 1648–1659 (2010).
Weismann, D. et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81 (2011). This study reveals a new homeostatic function of factor H with important implications for curbing inflammation in the retina and other tissues.
Jourde-Chiche, N. et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 15, 87–108 (2019).
Mastellos, D. C., Reis, E. S., Ricklin, D., Smith, R. J. & Lambris, J. D. Complement C3-targeted therapy: replacing long-held assertions with evidence-based discovery. Trends Immunol. 38, 383–394 (2017).
Smith, R. J. H. et al. C3 glomerulopathy — understanding a rare complement-driven renal disease. Nat. Rev. Nephrol. 15, 129–143 (2019).
Nester, C. M. et al. Atypical aHUS: state of the art. Mol. Immunol. 67, 31–42 (2015).
Perkins, S. J. Genetic and protein structural evaluation of atypical hemolytic uremic syndrome and C3 glomerulopathy. Adv. Chronic Kidney Dis. 27, 120–127.e4 (2020).
Sinha, A., Singh, A. K., Kadni, T. S., Mullick, J. & Sahu, A. Virus-encoded complement regulators: current status. Viruses 13, 208 (2021).
Kumar, J. et al. Species specificity of vaccinia virus complement control protein for the bovine classical pathway is governed primarily by direct interaction of its acidic residues with factor I. J. Virol. 91, 668–685 (2017).
Agrawal, P., Nawadkar, R., Ojha, H., Kumar, J. & Sahu, A. Complement evasion strategies of viruses: an overview. Front. Microbiol. 8, 1117 (2017).
Chen, J. Y., Cortes, C. & Ferreira, V. P. Properdin: a multifaceted molecule involved in inflammation and diseases. Mol. Immunol. 102, 58 (2018).
Zhang, J. et al. Soluble collectin-12 mediates C3-independent docking of properdin that activates the alternative pathway of complement. eLlife 9, e60908 (2020).
Narni-Mancinelli, E. et al. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci. Immunol. 2, PMC5419422 (2017).
Geisbrecht, B. V., Lambris, J. D. & Gros, P. Complement component C3: a structural perspective and potential therapeutic implications. Semin. Immunol. 59, 101627 (2022).
Sundsmo, J. S. The leukocyte complement system. Fed. Proc. 41, 3094–3098 (1982). First report on the presence of a cellular complement system in lymphocytes that may mediate homeostatic functions beyond pathogen immunosurveillance.
Lambris, J. D., Dobson, N. J. & Ross, G. D. Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J. Exp. Med. 152, 1625–1644 (1980).
King, B. C. & Blom, A. M. Intracellular complement: evidence, definitions, controversies, and solutions. Immunol. Rev. 313, 104–119 (2023). Comprehensive overview of the mechanisms and functions of intracellular complement, which also highlights experimental and conceptual challenges.
Elvington, M., Liszewski, M. K., Bertram, P., Kulkarni, H. S. & Atkinson, J. P. A C3(H20) recycling pathway is a component of the intracellular complement system. J. Clin. Invest. 127, 970–981 (2017).
Tam, J. C. H., Bidgood, S. R., McEwan, W. A. & James, L. C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345, 1256070 (2014). This study reports how C3-tagging of viral and bacterial pathogens mediates intracellular antiviral immune responses.
Bottermann, M. et al. Complement C4 prevents viral infection through capsid inactivation. Cell Host Microbe 25, 617–629.e7 (2019).
Foss, S. et al. Potent TRIM21 and complement-dependent intracellular antiviral immunity requires the IgG3 hinge. Sci. Immunol. 7, eabj1640 (2022).
West, E. E., Afzali, B. & Kemper, C. Unexpected roles for intracellular complement in the regulation of Th1 responses. Adv. Immunol. 138, 35–70 (2018).
Kolev, M. et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity 42, 1033–1047 (2015).
Arbore, G. et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science 352, aad1210 (2016). This study provides important biological insight into the crosstalk of complement with innate immune signalling pathways driving T cell trajectories and effector functions.
Cardone, J. et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 11, 862–871 (2010).
Arbore, G. et al. Complement receptor CD46 co-stimulates optimal human CD8+ T cell effector function via fatty acid metabolism. Nat. Commun. 9, 4186 (2018).
Kolev, M. et al. Diapedesis-induced integrin signaling via LFA-1 facilitates tissue immunity by inducing intrinsic complement C3 expression in immune cells. Immunity 52, 513–527.e8 (2020).
Dutta, K., Friscic, J. & Hoffmann, M. H. Targeting the tissue-complosome for curbing inflammatory disease. Semin. Immunol. 60, 101644 (2022).
Niyonzima, N. et al. Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Sci. Immunol. 6, eabf2489 (2021).
Friščić, J. et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54, 1002–1021.e10 (2021). This study reveals important mechanistic insights into how complement C3 signalling in fibroblasts drives inflammatory tissue priming.
King, B. C. et al. Complement component C3 is highly expressed in human pancreatic islets and prevents β cell death via ATG16L1 interaction and autophagy regulation. Cell Metab. 29, 202–210.e6 (2019).
Kremlitzka, M. et al. Alternative translation and retrotranslocation of cytosolic C3 that detects cytoinvasive bacteria. Cell. Mol. Life Sci. 79, 291 (2022).
Golec, E. et al. Alternative splicing encodes functional intracellular CD59 isoforms that mediate insulin secretion and are down-regulated in diabetic islets. Proc. Natl Acad. Sci. USA 119, e2120083119 (2022). This study describes a novel intracellular function of CD59 variants and links their dysregulation to insulin imbalance in type 2 diabetes.
Chehoud, C. et al. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc. Natl Acad. Sci. USA 110, 15061–15066 (2013).
Howden, B. P. et al. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 21, 380–395 (2023).
Garcia, B. L., Ramyar, K. X., Ricklin, D., Lambris, J. D. & Geisbrecht, B. V. Advances in understanding the structure, function, and mechanism of the SCIN and Efb families of Staphylococcal immune evasion proteins. Adv. Exp. Med. Biol. 946, 113–133 (2012).
Rooijakkers, S. H. et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat. Immunol. 10, 721–727 (2009).
Chen, H. et al. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc. Natl Acad. Sci. USA 107, 17621–17626 (2010).
Hammel, M. et al. A structural basis for complement inhibition by Staphylococcus aureus. Nat. Immunol. 8, 430–437 (2007). This study provides a structural basis for understanding how a microbial immune evasion protein induces conformational changes in C3 that prevent its proteolytic activation.
Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 35, 3–11 (2014).
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759 (2018).
Hajishengallis, G. & Lambris, J. D. Complement and dysbiosis in periodontal disease. Immunobiology 217, 1111–1116 (2012).
Lamont, R. J. & Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 21, 172–183 (2015).
Kajikawa, T. et al. C3-targeted host-modulation approaches to oral inflammatory conditions. Semin. Immunol. 59, 101608 (2022).
Potempa, M. & Potempa, J. Protease-dependent mechanisms of complement evasion by bacterial pathogens. Biol. Chem. 393, 873–888 (2012).
Hajishengallis, G. et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506 (2011). This study provides biological insight into how complement fuels the dysbiotic transformation of a commensal microbiota driving periodontal inflammation.
Maekawa, T. et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 15, 768–778 (2014).
Makkawi, H. et al. Porphyromonas gingivalis stimulates TLR2-PI3K signaling to escape immune clearance and induce bone resorption independently of MyD88. Front. Cell. Infect. Microbiol. 7, 359 (2017).
Liang, S. et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J. Immunol. 186, 869–877 (2011).
Maekawa, T. et al. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J. Immunol. 192, 6020–6027 (2014). This study provides the first preclinical proof of concept for therapeutic targeting of complement C3 in periodontal disease.
Maekawa, T. et al. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J. Clin. Periodontol. 43, 238–249 (2016).
Kajikawa, T. et al. Safety and efficacy of the complement inhibitor AMY-101 in a natural model of periodontitis in non-human primates. Mol. Ther. Methods Clin. Dev. 6, 207–215 (2017).
Dutzan, N. et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 10, eaat0797 (2018).
Wang, H. et al. Complement is required for microbe-driven induction of Th17 and periodontitis. J. Immunol. 209, 1370–1378 (2022).
Hajishengallis, G. & Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440 (2021).
Li, X. et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185, 1709–1727.e18 (2022).
Mastellos, D. C., Deangelis, R. A. & Lambris, J. D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 25, 29–38 (2013).
Stephan, A. H., Barres, B. A. & Stevens, B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 35, 369–389 (2012). Comprehensive overview of the roles of complement in synaptic pruning and microglial activation in both health and disease.
Deangelis, R. A. et al. A complement-IL-4 regulatory circuit controls liver regeneration. J. Immunol. 188, 641–648 (2012).
Haynes, T. et al. Complement anaphylatoxin C3a is a potent inducer of embryonic chick retina regeneration. Nat. Commun. 4, 2312 (2013).
Kimura, Y. et al. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 170, 2331–2339 (2003).
Tsonis, P. A., Lambris, J. D. & Del Rio-Tsonis, K. To regeneration… with complement. Adv. Exp. Med. Biol. 586, 63–70 (2006).
Strey, C. W. et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 198, 913–923 (2003). This study documents the crosstalk of complement signalling with cytokine-driven pathways in promoting mammalian liver regeneration.
Markiewski, M. M. et al. The regulation of liver cell survival by complement. J. Immunol. 182, 5412–5418 (2009).
He, S. et al. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J. Clin. Invest. 119, 2304–2316 (2009).
Carmona-Fontaine, C. et al. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev. Cell 21, 1026–1037 (2011). This study reveals a fundamental role of the complement C3a–C3aR axis in early embryonic development.
Broders-Bondon, F. et al. Control of the collective migration of enteric neural crest cells by the complement anaphylatoxin C3a and N-cadherin. Dev. Biol. 414, 85–99 (2016).
Rahpeymai, Y. et al. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J. 25, 1364–1374 (2006).
Coulthard, L. G., Hawksworth, O. A., Conroy, J., Lee, J. D. & Woodruff, T. M. Complement C3a receptor modulates embryonic neural progenitor cell proliferation and cognitive performance. Mol. Immunol. 101, 176–181 (2018).
Coulthard, L. G. et al. Complement C5aR1 signaling promotes polarization and proliferation of embryonic neural progenitor cells through PKCζ. J. Neurosci. 37, 5395–5407 (2017).
Taylor, R. P. & Lindorfer, M. A. Cytotoxic mechanisms of immunotherapy: harnessing complement in the action of anti-tumor monoclonal antibodies. Semin. Immunol. 28, 309–316 (2016).
Reis, E. S., Mastellos, D. C., Ricklin, D., Mantovani, A. & Lambris, J. D. Complement in cancer: untangling an intricate relationship. Nat. Rev. Immunol. 18, 5–18 (2018).
Roumenina, L. T., Daugan, M. V., Petitprez, F., Sautès-Fridman, C. & Fridman, W. H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019).
Oostindie, S. C. et al. DuoHexaBody-CD37®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies. Blood Cancer J. 10, 30 (2020).
Ostrand-Rosenberg, S. Cancer and complement. Nat. Biotechnol. 26, 1348–1349 (2008).
Markiewski, M. M. et al. Modulation of the antitumor immune response by complement. Nat. Immunol. 9, 1225–1235 (2008). This study provides the first evidence for a tumour-promoting role of complement via myeloid cell immunosuppression in a model of cervical cancer.
Ajona, D. et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 7, 694–703 (2017). This study provided the first therapeutic proof-of-concept for combined blockade of complement and immune-checkpoint signalling in cancer.
Pio, R., Ajona, D., Ortiz-Espinosa, S., Mantovani, A. & Lambris, J. D. Complementing the cancer-immunity cycle. Front. Immunol. 10, 774 (2019).
Ajona, D., Ortiz-Espinosa, S. & Pio, R. Complement anaphylatoxins C3a and C5a: emerging roles in cancer progression and treatment. Semin. Cell Dev. Biol. 85, 153–163 (2019).
Vadrevu, S. K. et al. Complement C5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 74, 3454–3465 (2014).
Guglietta, S. et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 7, 11037 (2016).
Kwak, J. W. et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 78, 143–156 (2018).
Su, S. et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16 (2018).
Magrini, E., Minute, L., Dambra, M. & Garlanda, C. Complement activation in cancer: effects on tumor-associated myeloid cells and immunosuppression. Semin. Immunol. 60, 101642 (2022).
Surace, L. et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity 42, 767–777 (2015).
Medler, T. R. et al. Complement C5a fosters squamous carcinogenesis and limits T cell response to chemotherapy. Cancer Cell 34, 561–578.e6 (2018).
Magrini, E. et al. Complement activation promoted by the lectin pathway mediates C3aR-dependent sarcoma progression and immunosuppression. Nat. Cancer 2, 218–232 (2021).
Sodji, Q. H. et al. The combination of radiotherapy and complement C3a inhibition potentiates natural killer cell functions against pancreatic cancer. Cancer Res. Commun. 2, 725–738 (2022).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019).
Daugan, M. V. et al. Intracellular factor H drives tumor progression independently of the complement cascade. Cancer Immunol. Res 9, 909–925 (2021). This study describes non-canonical functions of intracellular factor H in cancer that are independent of its complement regulatory activity.
Roumenina, L. T. et al. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol. Res. 7, 1091–1105 (2019).
Kulasekararaj, A. G. et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor–experienced adult patients with PNH: the 302 study. Blood 133, 540–549 (2019).
Petrisko, T. J., Gomez-Arboledas, A. & Tenner, A. J. Complement as a powerful ‘influencer’ in the brain during development, adulthood and neurological disorders. Adv. Immunol. 152, 157–222 (2021).
Lee, J. D., Coulthard, L. G. & Woodruff, T. M. Complement dysregulation in the central nervous system during development and disease. Semin. Immunol. 45, 101340 (2019).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Parker, S. E., Bellingham, M. C. & Woodruff, T. Complement drives circuit modulation in the adult brain.Prog. Neurobiol. 214, 102282 (2022).
Zelek, W. M. & Morgan, B. P. Targeting complement in neurodegeneration: challenges, risks, and strategies. Trends Pharmacol. Sci. 43, 615–628 (2022).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Stokowska, A. et al. Complement C3a treatment accelerates recovery after stroke via modulation of astrocyte reactivity and cortical connectivity. J. Clin. Invest. 133, e162253 (2023).
Vasek, M. J. et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 (2016).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Kalinowski, A. et al. Increased activation product of complement 4 protein in plasma of individuals with schizophrenia. Transl. Psychiatry 11, 486 (2021).
Yilmaz, M. et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat. Neurosci. 24, 214–224 (2021).
Litvinchuk, A. et al. Complement C3aR inactivation attenuates tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer’s disease. Neuron 100, 1337–1353.e5 (2018).
Yang, H. et al. Mechanistic insight into female predominance in Alzheimer’s disease based on aberrant protein S-nitrosylation of C3. Sci. Adv. 8, eade0764 (2022). This study links complement-driven synaptic loss and cognitive decline in Alzheimer disease with female hormonal regulation of post-translational modifications on C3.
Lambris, J. D., Rosenthal, A., Pelegrín, P., Tremblay, M. È. & Chen, P. The future of neuroimmunology research for CNS disease therapeutics. Trends Pharmacol. Sci. 43, 611–614 (2022).
Ekdahl, K. N., Soveri, I., Hilborn, J., Fellstrom, B. & Nilsson, B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat. Rev. Nephrol. 13, 285–296 (2017).
Mastellos, D. C., Ricklin, D. & Lambris, J. D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 18, 707–729 (2019).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Risitano, A. M., Peffault de Latour, R., Marano, L. & Frieri, C. Discovering C3 targeting therapies for paroxysmal nocturnal hemoglobinuria: achievements and pitfalls. Semin. Immunol. 59, 101618 (2022).
Takeda, J. et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 73, 703–711 (1993).
Mastellos, D. C., Ricklin, D., Yancopoulou, D., Risitano, A. & Lambris, J. D. Complement in paroxysmal nocturnal hemoglobinuria: exploiting our current knowledge to improve the treatment landscape. Expert Rev. Hematol. 7, 583–598 (2014).
Risitano, A. M. et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood 113, 4094–4100 (2009).
Lin, Z. et al. Complement C3dg-mediated erythrophagocytosis: implications for paroxysmal nocturnal hemoglobinuria. Blood 126, 891–894 (2015).
Risitano, A. M. et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood 123, 2094–2101 (2014). This study provides the first therapeutic proof of concept for the broader efficacy of C3 modulation in PNH.
Merle, N. S., Boudhabhay, I., Leon, J., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement activation during intravascular hemolysis: implication for sickle cell disease and hemolytic transfusion reactions. Transfus. Clin. Biol. 26, 116–124 (2019).
Frimat, M. et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 122, 282–292 (2013).
Merle, N. S. et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 3, e96910 (2018).
Roumenina, L. T., Bartolucci, P. & Pirenne, F. The role of complement in post-transfusion hemolysis and hyperhemolysis reaction. Transfus. Med. Rev. 33, 225–230 (2019).
Gerogianni, A. et al. Heme interferes with complement factor I-dependent regulation by enhancing alternative pathway activation. Front. Immunol. 13, 901876 (2022).
Merle, N. S. et al. P-selectin drives complement attack on endothelium during intravascular hemolysis in TLR-4/heme-dependent manner. Proc. Natl Acad. Sci. USA 116, 6280–6285 (2019).
van Lookeren, C. M., Strauss, E. C. & Yaspan, B. L. Age-related macular degeneration: complement in action. Immunobiology 221, 733–739 (2016).
Kim, B. J., Mastellos, D. C., Li, Y., Dunaief, J. L. & Lambris, J. D. Targeting complement components C3 and C5 for the retina: key concepts and lingering questions. Prog. Retin. Eye Res. 83, 100936 (2021).
Nozaki, M. et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl Acad. Sci. USA 103, 2328–2333 (2006).
Alic, L. et al. A genome-wide association study identifies key modulators of complement factor H binding to malondialdehyde-epitopes. Proc. Natl Acad. Sci. USA 117, 9942–9951 (2020).
Tzoumas, N. et al. Rare complement factor I variants associated with reduced macular thickness and age-related macular degeneration in the UK Biobank. Hum. Mol. Genet. 31, 2678–2692 (2022).
Hageman, G. S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl Acad. Sci. USA 102, 7227–7232 (2005). This is one of the first studies implicating complement alternative pathway dysregulation and a common genetic polymorphism in factor H that predisposes to AMD.
Haines, J. L. et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421 (2005).
Clark, S. J. et al. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J. Immunol. 190, 2049–2057 (2013).
Calippe, B. et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity 46, 261–272 (2017).
Cerniauskas, E. et al. Complement modulation reverses pathology in Y402H-retinal pigment epithelium cell model of age-related macular degeneration by restoring lysosomal function. Stem Cell Transl. Med. 9, 1585–1603 (2020).
Armento, A. et al. CFH loss in human RPE cells leads to inflammation and complement system dysregulation via the NF-κB pathway. Int. J. Mol. Sci. 22, 8727 (2021).
Armento, A. et al. Loss of complement factor H impairs antioxidant capacity and energy metabolism of human RPE cells. Sci. Rep. 10, 10320 (2020).
Cipriani, V. et al. Increased circulating levels of Factor H-related protein 4 are strongly associated with age-related macular degeneration. Nat. Commun. 11, 778 (2020).
Katschke, K. J. et al. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci. Rep. 8, 7348 (2018).
Finkel, Y. et al. SARS-CoV-2 uses a multipronged strategy to impede host protein synthesis. Nature 594, 240–245 (2021).
Diamond, M. S., Lambris, J. D., Ting, J. P. & Tsang, J. S. Considering innate immune responses in SARS-CoV-2 infection and COVID-19. Nat. Rev. Immunol. 22, 465–470 (2022).
Risitano, A. M. et al. Complement as a target in COVID-19? Nat. Rev. Immunol. 20, 343–344 (2020).
Ramlall, V. et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 26, 1609–1615 (2020). This study used transcriptomics and genetic association analyses to link complement and coagulation-related loci to COVID-19 susceptibility and severity.
Skendros, P. et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 130, 6151–6157 (2020).
Java, A. et al. The complement system in COVID-19: friend and foe? JCI Insight 5, e140711 (2020).
Afzali, B., Noris, M., Lambrecht, B. N. & Kemper, C. The state of complement in COVID-19. Nat. Rev. Immunol. 22, 77–84 (2022).
Holter, J. C. et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl Acad. Sci. USA 117, 25018–25025 (2020).
Casciola-Rosen, L. et al. IgM anti-ACE2 autoantibodies in severe COVID-19 activate complement and perturb vascular endothelial function. JCI insight 7, e158362 (2022).
Stravalaci, M. et al. Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules. Nat. Immunol. 23, 275–286 (2022).
Yu, J. et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 136, 2080–2089 (2020).
Yan, B. et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci. Immunol. 6, eabg0833 (2021).
Anders, H. J., Kitching, A. R., Leung, N. & Romagnani, P. Glomerulonephritis: immunopathogenesis and immunotherapy. Nat. Rev. Immunol. 23, 453–471 (2023).
Bomback, A. S. et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 7, 748–756 (2012).
Tatapudi, V. S. & Montgomery, R. A. Therapeutic modulation of the complement system in kidney transplantation: clinical indications and emerging drug leads. Front. Immunol. 10, 2306 (2019).
Mannes, M. et al. Complement C3 activation in the ICU: disease and therapy as Bonnie and Clyde. Semin. Immunol. 60, 101640 (2022).
Boudhabhay, I. et al. Complement activation is a crucial driver of acute kidney injury in rhabdomyolysis. Kidney Int. 99, 581–597 (2021).
Thurman, J. M., Frazer-Abel, A. & Holers, V. M. The evolving landscape for complement therapeutics in rheumatic and autoimmune diseases. Arthritis Rheumatol. 69, 2102–2113 (2017).
Banda, N. K. et al. Analysis of complement gene expression, clinical associations, and biodistribution of complement proteins in the synovium of early rheumatoid arthritis patients reveals unique pathophysiologic features. J. Immunol. 208, 2482–2496 (2022).
Schmitz, R. et al. C3 complement inhibition prevents antibody-mediated rejection and prolongs renal allograft survival in sensitized non-human primates. Nat. Commun. 12, 5456 (2021).
Ronco, P. et al. Membranous nephropathy.Nat. Rev. Dis. Primers 7, 69 (2021).
Seifert, L. et al. The classical pathway triggers pathogenic complement activation in membranous nephropathy. Nat. Commun. 14, 473 (2023).
Haddad, G. et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J. Clin. Invest. 131, e140453 (2021).
Mastellos, D. C. et al. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin. Immunol. 220, 108598 (2020).
Vlaar, A. P. J. et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 10, 375 (2022).
Risitano, A. M. et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open-label, single-arm, phase 2, proof-of-concept trial. Lancet Haematol. 8, e344–e354 (2021).
Kulasekararaj, A. G. et al. Phase 2 study of danicopan in patients with paroxysmal nocturnal hemoglobinuria with an inadequate response to eculizumab. Blood 138, 1928–1938 (2021).
Nishimura, J. et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 370, 632–639 (2014).
Röth, A. et al. Sutimlimab in cold agglutinin disease. N. Engl. J. Med. 384, 1323–1334 (2021).
Holers, V. M., Rohrer, B. & Tomlinson, S. CR2-mediated targeting of complement inhibitors: bench-to-bedside using a novel strategy for site-specific complement modulation. Adv. Exp. Med. Biol. 735, 137–154 (2013).
FDA. Approves SYFOVRETM (pegcetacoplan injection) as the First and Only Treatment for Geographic Atrophy (GA), a Leading Cause of Blindness https://investors.apellis.com/news-releases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only (2023).
BUSINESS WIRE. Iveric Bio Announces Positive Topline Data from Zimura® GATHER2 Phase 3 Clinical Trial in Geographic Atrophy https://www.businesswire.com/news/home/20220905005451/en/Iveric-Bio-Announces-Positive-Topline-Data-from-Zimura®-GATHER2-Phase-3-Clinical-Trial-in-Geographic-Atrophy (2022).
Jevsevar, S., Kunstelj, M. & Porekar, V. G. PEGylation of therapeutic proteins. Biotechnol. J. 5, 113–128 (2010).
Schnabolk, G. et al. Delivery of CR2-fH using AAV vector therapy as treatment strategy in the mouse model of choroidal neovascularization. Mol. Ther. Methods Clin. Dev. 9, 1–11 (2018).
Hasturk, H., Hajishengallis, G., Lambris, J. D., Mastellos, D. C. & Yancopoulou, D. Phase IIa clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J. Clin. Invest 131, e152973 (2021). This study reports on the clinical efficacy of a C3-targeted complement inhibitor in a phase II trial in individuals with periodontal inflammation.
Fridkis-Hareli, M. et al. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood 118, 4705–4713 (2011).
Atkinson, C. et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation 131, 1171–1180 (2015).
Alawieh, A., Langley, E. F. & Tomlinson, S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci. Transl. Med. 10, eaao6459 (2018).
Wu, Y. Q. et al. Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine. J. Immunol. 186, 4269–4277 (2011).
Bechtler, C. et al. Complement-regulatory biomaterial coatings: activity and selectivity profile of the factor H-binding peptide 5C6. Acta Biomater. 155, 123–138 (2023).
Schmidt, C. Q. et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J. Immunol. 190, 5712–5721 (2013).
Pittock, S. J. et al. Eculizumab in aquaporin-4–positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 381, 614–625 (2019).
Batista, A. F. et al. Complement C3 lowering in adult inducible conditional knockout mice: long-lasting effects. Alzheimers Dement. 18, e068094 (2022).
Desai, J. V. et al. C5a-licensed phagocytes drive sterilizing immunity during systemic fungal infection. Cell 186, 2802–2822.e22 (2023).
Lamers, C., Mastellos, D. C., Ricklin, D. & Lambris, J. D. Compstatins: the dawn of clinical C3-targeted complement inhibition. Trends Pharmacol. Sci. 43, 629–640 (2022).
Mastellos, D. C. & Lambris, J. D. Recent developments in C3-targeted complement therapeutics. Semin. Immunol. 60, 101645 (2022).
Willrich, M. A. V., Braun, K. M. P., Moyer, A. M., Jeffrey, D. H. & Frazer-Abel, A. Complement testing in the clinical laboratory. Crit. Rev. Clin. Lab. Sci. 58, 447–478 (2021).
Harris, C. L., Heurich, M., Rodriguez de Cordoba, S. & Morgan, B. P. The complotype: dictating risk for inflammation and infection. Trends Immunol. 33, 513–521 (2012).
Gaya da Costa, M. et al. Age and sex-associated changes of complement activity and complement levels in a healthy caucasian population. Front. Immunol. 9, 2664 (2018). The results of this study underscore the need for considering sex-based differences in basal complement activity when conducting human trials with complement inhibitors.
Kamitaki, N. et al. Complement genes contribute sex-biased vulnerability in diverse disorders. Nature 582, 577–581 (2020).
Sahu, S. K. et al. Lung epithelial cell-derived C3 protects against pneumonia-induced lung injury.Sci. Immunol. 8, eabp9547 (2023).
Mastellos, D. C. et al. ‘Stealth’ corporate innovation: an emerging threat for therapeutic drug development. Nat. Immunol. 20, 1409–1413 (2019).
Pinto, M. R., Melillo, D., Giacomelli, S., Sfyroera, G. & Lambris, J. D. Ancient origin of the complement system: emerging invertebrate models. Adv. Exp. Med. Biol. 598, 372–388 (2007).
Nonaka, M. Evolution of the complement system. Subcell. Biochem. 80, 31–43 (2014).
Moore, S. R., Menon, S. S., Cortes, C. & Ferreira, V. P. Hijacking factor H for complement immune evasion. Front. Immunol. 12, 602277 (2021).
Shokal, U. & Eleftherianos, I. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front. Immunol. 8, 759 (2017).
Elvington, M., Liszewski, M. K. & Atkinson, J. P. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol. Rev. 274, 9 (2016).
Sunyer, J. O., Zarkadis, I. K., Sahu, A. & Lambris, J. D. Multiple forms of complement C3 in trout that differ in binding to complement activators. Proc. Natl Acad. Sci. USA 93, 8546–8551 (1996). This study was the first to show that teleost fish possess multiple C3 isoforms that endow these species with broad innate immune recognition capacity.
Luzzatto, L., Risitano, A. M. & Notaro, R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica 95, 523–526 (2010).
Frei, Y., Lambris, J. D. & Stockinger, B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol. Cell Probes 1, 141–149 (1987).
Garred, P., Tenner, A. J. & Mollnes, T. E. Therapeutic targeting of the complement system: from rare diseases to pandemics. Pharmacol. Rev. 73, 792–827 (2021).
Lee, J. W. et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood 133, 530–539 (2019).
Mastellos, D. C., Ricklin, D., Sfyroera, G. & Sahu, A. From discovery to approval: a brief history of the compstatin family of complement C3 inhibitors. Clin. Immunol. 235, 108785 (2022).
Katragadda, M., Magotti, P., Sfyroera, G. & Lambris, J. D. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 49, 4616–4622 (2006). This study reports the structure-guided design and activity of the compstatin-based C3 inhibitor that forms the basis of the currently approved drug Empaveli.
Mullard, A. First approval of a complement C3 inhibitor opens up autoimmune and inflammatory opportunities. Nat. Rev. Drug Discov. 20, 496 (2021).
Röth, A. et al. Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood 140, 980–991 (2022).
Jayne, D. R. W., Merkel, P. A., Schall, T. J. & Bekker, P. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
Schröder-Braunstein, J. & Kirschfink, M. Complement deficiencies and dysregulation: pathophysiological consequences, modern analysis, and clinical management. Mol. Immunol. 114, 299–311 (2019).
Pickering, M. C., Botto, M., Taylor, P. R., Lachmann, P. J. & Walport, M. J. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 76, 227–324 (2000).
Hajishengallis, G. et al. Complement inhibition in pre-clinical models of periodontitis and prospects for clinical application. Semin. Immunol. 28, 285–291 (2016).
Berger, N. et al. New analogs of the complement C3 inhibitor compstatin with increased solubility and improved pharmacokinetic profile. J. Med. Chem. 61, 6153–6162 (2018).
Hughes, S. et al. Prolonged intraocular residence and retinal tissue distribution of a fourth-generation compstatin-based C3 inhibitor in non-human primates. Clin. Immunol. 214, 108391 (2020).
Lamers, C. et al. Insight into mode-of-action and structural determinants of the 2 compstatin family of clinical complement inhibitors. Nat. Commun. 13, 5519 (2022).
Pedersen, H. et al. A C3-specific nanobody that blocks all three activation pathways in the human and murine complement system. J. Biol. Chem. 295, 8746–8758 (2020).
Acknowledgements
This work was supported by the Dr Ralph and Sallie Weaver Professorship of Research Medicine to J.D.L. and by a grant from the US National Institutes of Health (DE026152 to G.H.).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors (including third-generation compstatin analogues such as AMY-101). J.D.L. is an inventor of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes, some of which are developed by Amyndas Pharmaceuticals. J.D.L. and G.H. have a joint patent that describes the use of complement inhibitors for therapeutic purposes in periodontitis. J.D.L. is also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals (namely 4(1MeW)7W/POT-4/APL-1 and PEGylated derivatives such as APL-2/pegcetacoplan/Empaveli/Aspaveli/Syfovre). D.C.M. has provided paid consulting services to 4D Molecular Therapeutics and Merck KGaA.
Peer review
Peer review information
Nature Reviews Immunology thanks Idris Boudhabhay, Claudia Kemper, Natalia Kunz, Xaria Li, Lubka Roumenina and Trent Woodruff for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mastellos, D.C., Hajishengallis, G. & Lambris, J.D. A guide to complement biology, pathology and therapeutic opportunity. Nat Rev Immunol 24, 118–141 (2024). https://doi.org/10.1038/s41577-023-00926-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00926-1
This article is cited by
-
Myasthenia gravis: the changing treatment landscape in the era of molecular therapies
Nature Reviews Neurology (2024)
-
Canonical and non-canonical roles of complement in atherosclerosis
Nature Reviews Cardiology (2024)
-
Translating B cell immunology to the treatment of antibody-mediated allograft rejection
Nature Reviews Nephrology (2024)