Abstract
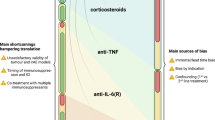
Immune-checkpoint inhibitors (ICIs) have dramatically changed the management of advanced cancers. Designed to enhance the antitumour immune response, they can also cause off-target immune-related adverse events (irAEs), which are sometimes severe. Although the efficacy of ICIs suggests that they could have wide-ranging benefits, clinical trials of the drugs have so far excluded patients with pre-existing autoimmune disease. However, evidence is accumulating with regard to the use of ICIs in this ‘at-risk’ population, with retrospective data suggesting that they have an acceptable safety profile, but that there is a risk of disease flare or other irAE occurrence. The management of immunosuppressive drugs at ICI initiation in patients with autoimmune disease (or later in instances of disease flare or irAE) remains a question of particular interest in clinical practice, in which there is always a search for the balance between protecting against autoimmunity and ensuring a good tumour response. Although temporary use of immunosuppressants seems safe, prolonged use or use at ICI initiation might hamper the antitumour immune response, prompting clinicians to use the minimal efficient immunosuppressive regimen. However, a new paradigm is emerging, in which inhibitors of TNF or IL-6 could have synergistic effects with ICIs on tumour response, while also preventing severe irAEs. If confirmed, this ‘decoupling’ effect on toxicity and efficacy could change therapeutic practice in this field. Knowledge of the current use of ICIs in patients with pre-existing autoimmune disease, particularly with regard to the use of immunosuppressive drugs and/or biologic DMARDs, can help to guide clinical practice.
Key points
-
Immune-checkpoint inhibitors (ICIs) should be offered to patients with pre-existing autoimmune disease who have advanced cancer, within the process of shared decision-making.
-
High risk of (generally mild) flare (up to 75%) is reported for patients with pre-existing rheumatoid arthritis, polymyalgia rheumatica and psoriatic arthritis following ICI initiation.
-
ICI-mediated immune toxicity is mostly manageable with glucocorticoids, and rarely requires DMARDs in patients with pre-existing autoimmune disease.
-
A minimal immunosuppressive regimen should be reached at ICI initiation, but selective therapies should be used for active and severe pre-existing autoimmune disease.
-
Basic and clinical research are needed to better understand the pathophysiology underlying ICI-induced autoimmune disease flare compared with immune-related adverse events, and to identify predictive factors of immune toxicity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Manson, G. et al. Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J. Immunother. Cancer 7, 337 (2019).
Abdel-Wahab, N., Shah, M., Lopez-Olivo, M. A. & Suarez-Almazor, M. E. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann. Intern. Med. 168, 121–130 (2018).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Nocturne, G. & Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 14, 133–145 (2018).
Franks, A. L. & Slansky, J. E. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer. Res. 32, 1119–1136 (2012).
Askling, J. et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann. Rheum. Dis. 64, 1414–1420 (2005).
Dreyer, L. et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides — a follow-up study from the DANBIO Registry. Ann. Rheum. Dis. 72, 79–82 (2013).
Mercer, L. K. et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 76, 497–503 (2017).
Mercer, L. K. et al. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 74, 1087–1093 (2015).
Seror, R. & Mariette, X. Malignancy and the risks of biologic therapies: current status. Rheum. Dis. Clin. North. Am. 43, 43–64 (2017).
Mariette, X. et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann. Rheum. Dis. 70, 1895–1904 (2011).
De Cock, D. & Hyrich, K. Malignancy and rheumatoid arthritis: epidemiology, risk factors and management. Best. Pract. Res. Clin. Rheumatol. 32, 869–886 (2018).
Lopez-Olivo, M. A. et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA 308, 898–908 (2012).
Ytterberg, S. R. et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 386, 316–326 (2022).
Manger, B. & Schett, G. Rheumatic paraneoplastic syndromes — a clinical link between malignancy and autoimmunity. Clin. Immunol. 186, 67–70 (2018).
Moinzadeh, P. et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res. Ther. 16, R53 (2014).
Joseph, C. G. et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014).
Yadav, S. et al. Autoantibodies as diagnostic and prognostic cancer biomarker: detection techniques and approaches. Biosens. Bioelectron. 139, 111315 (2019).
Gajewski, T. F., Fuertes, M., Spaapen, R., Zheng, Y. & Kline, J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 23, 286–292 (2011).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Matsumura, N., Ohtsuka, M., Kikuchi, N. & Yamamoto, T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm. Venereol. 96, 259–260 (2016).
Huang, C. et al. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J. Autoimmun. 104, 102333 (2019).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362 (2021).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Hirsch, L., Zitvogel, L., Eggermont, A. & Marabelle, A. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 120, 3–5 (2019).
Wolchok, J. D. et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 40, 127–137 (2022).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Yoest, J. M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 6, 73–82 (2017).
Hu, W., Wang, G., Wang, Y., Riese, M. J. & You, M. Uncoupling therapeutic efficacy from immune-related adverse events in immune checkpoint blockade. iScience 23, 101580 (2020).
Abdel-Wahab, N., Shah, M. & Suarez-Almazor, M. E. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 11, e0160221 (2016).
Belkhir, R. et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann. Rheum. Dis. 76, 1747–1750 (2017).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016).
Horvat, T. Z. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 33, 3193–3198 (2015).
Warner, B. M. et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 24, 1259–1269 (2019).
Carbonnel, F. et al. Inflammatory bowel disease and cancer response due to anti-CTLA-4: is it in the flora? Semin. Immunopathol. 39, 327–331 (2017).
Calabrese, L. H., Calabrese, C. & Cappelli, L. C. Rheumatic immune-related adverse events from cancer immunotherapy. Nat. Rev. Rheumatol. 14, 569–579 (2018).
Brahmer, J. R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9, e002435 (2021).
Jing, Y. et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat. Commun. 11, 4946 (2020).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Ribas, A. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16, 908–918 (2015).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Subudhi, S. K. et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl Acad. Sci. USA 113, 11919–11924 (2016).
Oh, D. Y. et al. Immune toxicities elicited by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 77, 1322–1330 (2017).
Lozano, A. X. et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. https://doi.org/10.1038/s41591-021-01623-z (2022).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Berner, F. et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5, 1043–1047 (2019).
Läubli, H. et al. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology 7, e1386362 (2018).
Kim, K. H. et al. Immune-related adverse events are clustered into distinct subtypes by T-cell profiling before and early after anti-PD-1 treatment. Oncoimmunology 9, 1722023 (2020).
Grigoriou, M. et al. Regulatory T-cell transcriptomic reprogramming characterizes adverse events by checkpoint inhibitors in solid tumors. Cancer Immunol. Res. 9, 726–734 (2021).
Gonugunta, A. S. et al. Humoral and cellular correlates of a novel immune-related adverse event and its treatment. J. Immunother. Cancer 9, e003585 (2021).
Reschke, R. et al. Distinct immune signatures indicative of treatment response and immune-related adverse events in melanoma patients under immune checkpoint inhibitor therapy. Int. J. Mol. Sci. 22, 8017 (2021).
Kim, S. T. et al. Distinct molecular and immune hallmarks of inflammatory arthritis induced by immune checkpoint inhibitors for cancer therapy. Nat. Commun. 13, 1970 (2022).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671.e22 (2020).
Wang, R. et al. High-dimensional analyses of checkpoint-inhibitor related arthritis synovial fluid cells reveal a unique, proliferating CD38hi cytotoxic CD8 T cell population induced by type I IFN [abstract]. Arthritis Rheumatol. 72 (Suppl. 10): abstract 1443 (2020).
Murray-Brown, W. et al. Nivolumab-induced synovitis is characterized by florid T cell infiltration and rapid resolution with synovial biopsy-guided therapy. J. Immunother. Cancer 8, e000281 (2020).
Das, R. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018).
Patel, A. J. et al. Regulatory B cell repertoire defects predispose lung cancer patients to immune-related toxicity following checkpoint blockade. Nat. Commun. 13, 3148 (2022).
de Moel, E. C. et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol. Res. 7, 6–11 (2019).
Huang, Y.-T., Chen, Y.-P., Lin, W.-C., Su, W.-C. & Sun, Y.-T. Immune checkpoint inhibitor-induced myasthenia gravis. Front. Neurol. 11, 634 (2020).
Kobayashi, T. et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J. Endocr. Soc. 2, 241–251 (2018).
Mammen, A. L. et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann. Rheum. Dis. 78, 150–152 (2019).
Tarhini, A. A. et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 3, 39 (2015).
Lim, S. Y. et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin. Cancer Res. 25, 1557–1563 (2019).
Husain, B. et al. Inflammatory markers in autoimmunity induced by checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 147, 1623–1630 (2021).
Wang, Y. N. et al. Elevated levels of IL-17A and IL-35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Oncol. Lett. 20, 611–622 (2020).
Khan, S. et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br. J. Cancer 120, 63–68 (2019).
Iwama, S. et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra45 (2014).
Cappelli, L. C., Dorak, M. T., Bettinotti, M. P., Bingham, C. O. & Shah, A. A. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology 58, 476–480 (2019).
Hasan Ali, O. et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 107, 8–14 (2019).
Wölffer, M. et al. Biomarkers associated with immune-related adverse events under checkpoint inhibitors in metastatic melanoma. Cancers 14, 302 (2022).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Johnson, D. B. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2, 234–240 (2016).
Lee, B. et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann. Oncol. 27, 1174–1177 (2016).
Menzies, A. M. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28, 368–376 (2017).
Gutzmer, R. et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur. J. Cancer 75, 24–32 (2017).
Danlos, F.-X. et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 91, 21–29 (2018).
Mitchell, E. L. et al. Rheumatic immune-related adverse events secondary to anti-programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: a case series. Eur. J. Cancer 105, 88–102 (2018).
Richter, M. D. et al. Brief report: cancer immunotherapy in patients with preexisting rheumatic disease: the Mayo Clinic experience. Arthritis Rheumatol. 70, 356–360 (2018).
Leonardi, G. C. et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J. Clin. Oncol. 36, 1905–1912 (2018).
Kähler, K. C. et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol. Immunother. 67, 825–834 (2018).
Cortellini, A. et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist 24, e327–e337 (2019).
Tison, A. et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 71, 2100–2111 (2019).
Martinez Chanza, N. et al. Safety and efficacy of immune checkpoint inhibitors in advanced urological cancers with pre-existing autoimmune disorders: a retrospective international multicenter study. J. Immunother. Cancer 8, e000538 (2020).
Abu-Sbeih, H. et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J. Clin. Oncol. 38, 576–583 (2020).
Loriot, Y. et al. Safety and efficacy of atezolizumab in patients with autoimmune disease: subgroup analysis of the SAUL study in locally advanced/metastatic urinary tract carcinoma. Eur. J. Cancer 138, 202–211 (2020).
Efuni, E. et al. Risk of toxicity after initiating immune checkpoint inhibitor treatment in patients with rheumatoid arthritis. J. Clin. Rheumatol. 27, 267–271 (2021).
Hoa, S. et al. Preexisting autoimmune disease and immune-related adverse events associated with anti-PD-1 cancer immunotherapy: a national case series from the Canadian Research Group of Rheumatology in Immuno-Oncology. Cancer Immunol. Immunother. 70, 2197–2207 (2021).
Tully, K. H. et al. Risk of immune-related adverse events in melanoma patients with preexisting autoimmune disease treated with immune checkpoint inhibitors: a population-based study using SEER-medicare data. Am. J. Clin. Oncol. 44, 413–418 (2021).
van der Kooij, M. K. et al. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease: a cohort study. Ann. Intern. Med. 174, 641–648 (2021).
Bhatlapenumarthi, V., Patwari, A. & Harb, A. J. Immune-related adverse events and immune checkpoint inhibitor tolerance on rechallenge in patients with irAEs: a single-center experience. J. Cancer Res. Clin. Oncol. 147, 2789–2800 (2021).
Yeung, C. et al. Safety and clinical outcomes of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune diseases. J. Immunother. 44, 362–370 (2021).
Panhaleux, M. et al. Anti-programmed death ligand 1 immunotherapies in cancer patients with pre-existing systemic sclerosis: a postmarketed phase IV safety assessment study. Eur. J. Cancer 160, 134–139 (2022).
Ansel, S., Rulach, R., Trotter, N. & Steele, N. Pembrolizumab for advanced non-small cell lung cancer (NSCLC): impact of autoimmune comorbidity and outcomes following treatment completion. J. Oncol. Pharm. Pract. https://doi.org/10.1177/10781552221079356 (2022).
Gulati, N. et al. Preexisting immune-mediated inflammatory disease is associated with improved survival and increased toxicity in melanoma patients who receive immune checkpoint inhibitors. Cancer Med. 10, 7457–7465 (2021).
Brown, L. J. et al. Combination anti-PD1 and ipilimumab therapy in patients with advanced melanoma and pre-existing autoimmune disorders. J. Immunother. Cancer 9, e002121 (2021).
Wu, C., Zhong, L., Wu, Q., Lin, S. & Xie, X. The safety and efficacy of immune-checkpoint inhibitors in patients with cancer and pre-existing autoimmune diseases. Immunotherapy 13, 527–539 (2021).
Kostine, M. et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 80, 36–48 (2021).
Klavdianou, K., Melissaropoulos, K., Filippopoulou, A. & Daoussis, D. Should we be afraid of immune check point inhibitors in cancer patients with pre-existing rheumatic diseases? Immunotherapy in pre-existing rheumatic diseases. Mediterr. J. Rheumatol. 32, 218–226 (2021).
Nishino, M., Giobbie-Hurder, A., Hatabu, H., Ramaiya, N. H. & Hodi, F. S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2, 1607–1616 (2016).
Jaberg-Bentele, N. F., Kunz, M., Abuhammad, S. & Dummer, R. Flare-up of rheumatoid arthritis by anti-CTLA-4 antibody but not by anti-PD1 therapy in a patient with metastatic melanoma. Case Rep. Dermatol. 9, 65–68 (2017).
Benson, Z., Gordon, S., Nicolato, P. & Poklepovic, A. Immunotherapy for metastatic melanoma with right atrial involvement in a patient with rheumatoid arthritis. Case Rep. Oncol. Med. 2017, 8095601 (2017).
Thomas, R., Patel, H. & Scott, J. Dermatomyositis flare with immune checkpoint inhibitor therapy for melanoma. Cureus 13, e14387 (2021).
Montfort, A. et al. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin. Cancer Res. 27, 1037–1047 (2021).
Ghosh, N. et al. Lower baseline autoantibody levels are associated with immune-related adverse events from immune checkpoint inhibition. J. Immunother. Cancer 10, e004008 (2022).
Sakakida, T. et al. Safety and efficacy of PD-1/PD-L1 blockade in patients with preexisting antinuclear antibodies. Clin. Transl. Oncol. 22, 919–927 (2020).
Toi, Y. et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 5, 376–383 (2019).
Wang, D. et al. Immune-related adverse events predict the efficacy of immune checkpoint inhibitors in lung cancer patients: a meta-analysis. Front. Oncol. 11, 631949 (2021).
Hussaini, S. et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors — a systematic review and meta-analysis. Cancer Treat. Rev. 92, 102134 (2021).
Teulings, H.-E. et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J. Clin. Oncol. 33, 773–781 (2015).
Yee, C. et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J. Exp. Med. 192, 1637–1644 (2000).
Chapman, N. M. & Chi, H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity 55, 14–30 (2022).
Arbour, K. C. et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J. Clin. Oncol. 36, 2872–2878 (2018).
Strehl, C. & Buttgereit, F. Optimized glucocorticoid therapy: teaching old drugs new tricks. Mol. Cell. Endocrinol. 380, 32–40 (2013).
Fucà, G. et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 4, e000457 (2019).
Draghi, A. et al. Differential effects of corticosteroids and anti-TNF on tumor-specific immune responses: implications for the management of irAEs. Int. J. Cancer 145, 1408–1413 (2019).
Brown, P. M., Pratt, A. G. & Isaacs, J. D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 12, 731–742 (2016).
Downey, S. G. et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 13, 6681–6688 (2007).
Schadendorf, D. et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J. Clin. Oncol. 35, 3807–3814 (2017).
Paz-Ares, L. G. et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 Trial. J. Thorac. Oncol. 17, 289–308 (2022).
Reck, M. et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 6, 100273 (2021).
Waterhouse, D. M. et al. Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non-small-cell lung cancer: CheckMate 153. J. Clin. Oncol. 38, 3863–3873 (2020).
Bilger, G. et al. Discontinuation of immune checkpoint inhibitor (ICI) above 18 months of treatment in real-life patients with advanced non-small cell lung cancer (NSCLC): INTEPI, a multicentric retrospective study. Cancer Immunol. Immunother. https://doi.org/10.1007/s00262-021-03114-z (2021).
Chatzidionysiou, K., Liapi, M., Tsakonas, G., Gunnarsson, I. & Catrina, A. Treatment of rheumatic immune-related adverse events due to cancer immunotherapy with immune checkpoint inhibitors — is it time for a paradigm shift? Clin. Rheumatol. 40, 1687–1695 (2021).
Chen, A. Y., Wolchok, J. D. & Bass, A. R. TNF in the era of immune checkpoint inhibitors: friend or foe? Nat. Rev. Rheumatol. 17, 213–223 (2021).
Bertrand, F. et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 8, 2256 (2017).
Hailemichael, Y. et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40, 509–523.e6 (2022).
Perez-Ruiz, E. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019).
Lesage, C. et al. Incidence and clinical impact of anti-TNFα treatment of severe immune checkpoint inhibitor-induced colitis in advanced melanoma: the Mecolit Survey. J. Immunother. 42, 175–179 (2019).
Wang, Y. et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J. Immunother. Cancer 6, 37 (2018).
Verheijden, R. J. et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 26, 2268–2274 (2020).
Zou, F. et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J. Immunother. Cancer 9, e003277 (2021).
Laino, A. S. et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 8, e000842 (2020).
Campochiaro, C. et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur. J. Intern. Med. 93, 87–94 (2021).
Weber, J. S. et al. 1040 O Phase II trial of ipilimumab, nivolumab and tocilizumab for unresectable metastatic melanoma. Ann. Oncol. 32, S869 (2021).
Lebbé, C. et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol. 37, 867–875 (2019).
Delyon, J. & Lebbe, C. IL-6 blockade in cancer patients treated with immune checkpoint blockade: a win-win strategy. Cancer Cell 40, 450–451 (2022).
Haanen, J. B. A. G. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv264–iv266 (2018).
Thompson, J. A. et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl Compr. Canc. Netw. 17, 255–289 (2019).
Kennedy, L. C., Bhatia, S., Thompson, J. A. & Grivas, P. Preexisting autoimmune disease: implications for immune checkpoint inhibitor therapy in solid tumors. J. Natl Compr. Canc. Netw. 17, 750–757 (2019).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021).
Haanen, J. et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 31, 724–744 (2020).
Michot, J.-M. et al. The 2016–2019 ImmunoTOX assessment board report of collaborative management of immune-related adverse events, an observational clinical study. Eur. J. Cancer 130, 39–50 (2020).
Naidoo, J. et al. A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J. Natl Compr. Canc. Netw. 17, 712–720 (2019).
Calabrese, L. & Mariette, X. The evolving role of the rheumatologist in the management of immune-related adverse events (irAEs) caused by cancer immunotherapy. Ann. Rheum. Dis. 77, 162–164 (2018).
Nabel, C. S. et al. Anti-PD-1 immunotherapy-induced flare of a known underlying relapsing vasculitis mimicking recurrent cancer. Oncologist 24, 1013–1021 (2019).
Yamada, T. et al. Non-small cell lung cancer treated by an anti-programmed cell death-1 antibody without a flare-up of preexisting granulomatosis with polyangiitis. Intern. Med. 58, 3129–3132 (2019).
Maul, L. V., Weichenthal, M., Kähler, K. C. & Hauschild, A. Successful anti-PD-1 antibody treatment in a metastatic melanoma patient with known severe autoimmune disease. J. Immunother. 39, 188–190 (2016).
Author information
Authors and Affiliations
Contributions
A.T., S.G., L.C., D.C. and M.K. researched data for the article and made substantial contributions to discussions of the content. A.T., S.G. and M.K. wrote the article. A.T., S.G., L.C., D.C. and M.K. contributed to reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.C. reports consultancy and receipt of an honorarium from Novartis and Bristol Myers Squibb. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks A. Bass, L. Cappelli and M. Suarez-Almazor for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tison, A., Garaud, S., Chiche, L. et al. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat Rev Rheumatol 18, 641–656 (2022). https://doi.org/10.1038/s41584-022-00841-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00841-0
This article is cited by
-
Antinuclear antibodies may predict the development of immune-related adverse events in asymptomatic patients treated with immune checkpoint inhibitors: results from a single-center cohort
Clinical and Experimental Medicine (2024)
-
MMP1, IL-1β, sTNFR-1, and IL-6 are prognostic factors for patients with unresectable or metastatic renal cell carcinoma treated with immune checkpoint inhibitors
International Journal of Clinical Oncology (2024)
-
Neuromyelitis optica associated with the use of Atezolizumab in a patient with advanced lung adenocarcinoma
Neurological Sciences (2024)
-
Prognostic significance of Lymphocyte-activation gene 3 (LAG3) in patients with solid tumors: a systematic review, meta-analysis and pan-cancer analysis
Cancer Cell International (2023)
-
Immunotherapy-induced thyroid dysfunction: an updated review
The Egyptian Journal of Internal Medicine (2023)