Abstract
The axial spondyloarthritis (axSpA) disease concept has undergone substantial change from when the entity ankylosing spondylitis was defined by the modified New York criteria in 1984. Developments in imaging, therapy and genetics have all contributed to changing the concept of axSpA from one of erosions in the sacroiliac joints to a spectrum of disease with and without changes evident on plain radiographs. Changes to the previously held concept and construct of the disease have also necessitated new classification criteria. The use of MRI, primarily of the sacroiliac joints, has substantially altered the diagnosis and differential diagnosis of axSpA. Many in the axSpA community believe that the current classification criteria lack specificity, and the CLASSIC study is underway to examine this area. Although much about the evolving axSpA disease concept is universally agreed, there remains disagreement about operationalizing aspects of it, such as the requirement for the objective demonstration of axial inflammation for the classification of axSpA. New imaging technologies, biomarkers and genetics data will probably necessitate ongoing revision of axSpA classification criteria. Advances in our knowledge of the biology of axSpA will settle some differences in opinion as to how the disease concept is applied to the classification and diagnosis of patients.
Key points
-
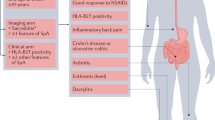
The concept of axial spondyloarthritis (axSpA) has expanded from ankylosing spondylitis with evidence of erosions to a spectrum of disease encompassing non-radiographic axSpA and radiographic axSpA.
-
The current classification criteria capture the entire spectrum of axSpA, but many in the field believe they lack specificity; the CLASSIC study is underway to further assess this issue.
-
The concept of axSpA is largely agreed upon in the research community, but opinion still diverges about some aspects, for example, the demonstration of objective axial inflammation for axSpA classification.
-
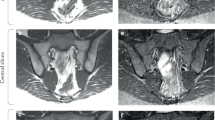
The current definition of a positive sacroiliac joint MRI scan lacks specificity for axSpA, as demonstrated in imaging studies of individuals with and without back pain and post-partum women.
-
Concepts such as the theory of natural kinds and latent class analysis enable us to further examine the crucial features of the axSpA concept, with sacroiliitis being the core feature.
-
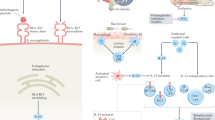
Advances in our understanding of the biology of axSpA via novel imaging, genetic and biomarker studies will probably enable the resolution of many current issues in axSpA diagnosis and classification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
de Winter, J. J. et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res. Ther. 18, 196 (2016).
Panush, R. S., Paraschiv, D. & Dorff, R. E. The tainted legacy of Hans Reiter. Semin. Arthritis Rheum. 32, 231–236 (2003).
Dougados, M. et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 34, 1218–1227 (1991).
Zochling, J., Brandt, J. & Braun, J. The current concept of spondyloarthritis with special emphasis on undifferentiated spondyloarthritis. Rheumatology 44, 1483–1491 (2005).
Moll, J. M. et al. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter’s disease, the intestinal arthropathies, and Behcet’s syndrome. Medicine 53, 343–364 (1974).
Kirino, Y. et al. Genome-wide association analysis identifies new susceptibility loci for Behcet’s disease and epistasis between HLA-B*51 and ERAP1. Nat. Genet. 45, 202–207 (2013).
International Genetics of Ankylosing Spondylitis Consortium (IGAS). et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738 (2013).
Wright, V. & Moll, J. The Meaning of Seronegativity and Seropositivity, in Seronegative Polyarthritis. (Elsevier/North-Holland Biomedical Press, 1976).
Nash, P. et al. Seronegative spondyloarthropathies: to lump or split? Ann. Rheum. Dis. 64, ii9–ii13 (2005).
Taylor, W. J. & Robinson, P. C. Classification criteria: peripheral spondyloarthropathy and psoriatic arthritis. Curr. Rheumatol. Rep. 15, 317 (2013).
Sieper, J. & Poddubnyy, D. Axial spondyloarthritis. Lancet 390, 73–84 (2017).
Taurog, J. D., Chhabra, A. & Colbert, R. A. Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med. 374, 2563–2574 (2016).
Olivieri, I. et al. Spondyloarthritis with onset after age 45. Curr. Rheumatol. Rep. 15, 374 (2013).
Toussirot, E. Late-onset ankylosing spondylitis and spondylarthritis: an update on clinical manifestations, differential diagnosis and pharmacological therapies. Drugs Aging 27, 523–531 (2010).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Poddubnyy, D. et al. Diagnostic accuracy of inflammatory back pain for axial spondyloarthritis in rheumatological care. RMD Open 4, e000825 (2018).
Hermann, K. G. et al. Magnetic resonance imaging of inflammatory lesions in the spine in ankylosing spondylitis clinical trials: is paramagnetic contrast medium necessary? J. Rheumatol. 32, 2056–2060 (2005).
de Hooge, M. et al. Magnetic resonance imaging of the sacroiliac joints in the early detection of spondyloarthritis: no added value of gadolinium compared with short tau inversion recovery sequence. Rheumatology 52, 1220–1224 (2013).
Maksymowych, W. P. et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann. Rheum. Dis. 78, 1550–1558 (2019).
Gong, Y. et al. Ten years’ experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum. 64, 1399–1406 (2012).
Bakker, P. A. et al. Is it useful to repeat MRI of the sacroiliac joints after three months or one year in the diagnostic process of patients with chronic back pain suspected of axial spondyloarthritis? Arthritis Rheumatol. 71, 382–391 (2019).
Sengupta, R. et al. Short-term repeat magnetic resonance imaging scans in suspected early axial spondyloarthritis are clinically relevant only in HLA-B27-positive male subjects. J. Rheumatol. 45, 202–205 (2018).
Rusman, T. et al. Presence of active MRI lesions in patients suspected of non-radiographic axial spondyloarthritis with high disease activity and chance at conversion after a 6-month follow-up period. Clin. Rheumatol. 39, 1521–1529 (2020).
van Onna, M. et al. HLA-B27 and gender independently determine the likelihood of a positive MRI of the sacroiliac joints in patients with early inflammatory back pain: a 2-year MRI follow-up study. Ann. Rheum. Dis. 70, 1981–1985 (2011).
Landewe, R. et al. A single determination of C-reactive protein does not suffice to declare a patient with a diagnosis of axial spondyloarthritis ‘CRP-negative’. Arthritis Res. Ther. 20, 209 (2018).
Visser, M. et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282, 2131–2135 (1999).
Aggarwal, R. et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res. 67, 891–897 (2015).
Khan, M. A. & van der Linden, S. Axial spondyloarthritis: a better name for an old disease: a step toward uniform reporting. ACR Open Rheumatol. 1, 336–339 (2019).
Rudwaleit, M., Khan, M. A. & Sieper, J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 52, 1000–1008 (2005).
Maksymowych, W. P. The role of imaging in the diagnosis and management of axial spondyloarthritis. Nat. Rev. Rheumatol. 15, 657–672 (2019).
Feldtkeller, E. et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol. Int. 23, 61–66 (2003).
Seven, S. et al. Magnetic resonance imaging of lesions in the sacroiliac joints for differentiation of patients with axial spondyloarthritis from control subjects with or without pelvic or buttock pain: a prospective, cross-sectional study of 204 participants. Arthritis Rheumatol. 71, 2034–2046 (2019).
de Winter, J. et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the Assessment of Spondyloarthritis International Society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 70, 1042–1048 (2018).
Baraliakos, X. et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. Ann. Rheum. Dis. 79, 186–192 (2020).
Varkas, G. et al. Effect of mechanical stress on magnetic resonance imaging of the sacroiliac joints: assessment of military recruits by magnetic resonance imaging study. Rheumatology 57, 508–513 (2018).
Weber, U. et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “background noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol. 70, 736–745 (2018).
Renson, T. et al. High prevalence of spondyloarthritis-like MRI lesions in postpartum women: a prospective analysis in relation to maternal, child and birth characteristics. Ann. Rheum. Dis. 79, 929–934 (2020).
Bennett, P. & Wood, P. Population studies of the rheumatic diseases; Proceedings of the Third International Symposium, New York, June 5th–10th, 1966. (Excerpta Medica Foundation, 1968)
Kellgren, J., Jeffrey, M. & Ball, J. The epidemiology of chronic rheumatism. Vol. 1 (Blackwell Scientific Publications, 1963).
Khan, M. A. et al. Spondylitic disease without radiologic evidence of sacroiliitis in relatives of HLA-B27 positive ankylosing spondylitis patients. Arthritis Rheum. 28, 40–43 (1985).
Sampaio-Barros, P. D. et al. Undifferentiated spondyloarthritis: a longterm followup. J. Rheumatol. 37, 1195–1199 (2010).
Poddubnyy, D. et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann. Rheum. Dis. 70, 1369–74 (2011).
Sany, J. et al. Unclassified HLA-B27 inflammatory rheumatic diseases: followup of 23 patients. Arthritis Rheum. 23, 258–259 (1980).
Schattenkirchner, M. & Kruger, K. Natural course and prognosis of HLA-B27-positive oligoarthritis. Clin. Rheumatol. 6, 83–86 (1987).
Mau, W. et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J. Rheumatol. 15, 1109–1114 (1988).
Oostveen, J. et al. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography. A prospective, longitudinal study. J. Rheumatol. 26, 1953–1958 (1999).
Bennett, A. N. et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum. 58, 3413–3418 (2008).
Dougados, M. et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 68, 1904–1913 (2016).
Dougados, M. et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann. Rheum. Dis. 76, 1823–1828 (2017).
Wang, R., Gabriel, S. E. & Ward, M. M. Progression of nonradiographic axial spondyloarthritis to ankylosing spondylitis: a population-based cohort study. Arthritis Rheumatol. 68, 1415–1421 (2016).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 68, 770–776 (2009).
van der Linden, S. et al. The ASAS criteria for axial spondyloarthritis: strengths, weaknesses, and proposals for a way forward. Curr. Rheumatol. Rep. 17, 62 (2015).
Thomas, G. P. et al. Genetic diagnostic profiling in axial spondyloarthritis: a real world study. Clin. Exp. Rheumatol. 35, 229–233 (2017).
Taylor, W. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 54, 2665–73 (2006).
Rindskopf, D. & Rindskopf, W. The value of latent class analysis in medical diagnosis. Stat. Med. 5, 21–27 (1986).
Sepriano, A. et al. What is axial spondyloarthritis? A latent class and transition analysis in the SPACE and DESIR cohorts. Ann. Rheum. Dis. 79, 324–331 (2020).
US National Library of Medicine. ClinicalTrials.gov. CLassification of axial spondyloarthritis inception cohort (CLASSIC). https://clinicaltrials.gov/ct2/show/NCT03993847 (2020).
Hayward, R. J. & Machado, P. M. Classification criteria in axial spondyloarthritis: what have we learned; where are we going? Rheum. Dis. Clin. North Am. 46, 259–274 (2020).
Dimitroulas, T. et al. Biologic drugs as analgesics for the management of low back pain and sciatica. Pain. Med. 20, 1678–1686 (2019).
Dougados, M. et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 66, 2091–2102 (2014).
Brown, M. A. et al. Evaluation of the effect of baseline MRI sacroiliitis and C reactive protein status on etanercept treatment response in non-radiographic axial spondyloarthritis: a post hoc analysis of the EMBARK study. Ann. Rheum. Dis. 77, 1091–1093 (2018).
Sieper, J. et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 67, 2702–2712 (2015).
Sieper, J. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822 (2013).
Pharmaceutical Benefits Advisory Committee. Public summary document — November 2017 PBAC meeting. http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2017-11/files/golimumab-nraxSpA-psd-november-2017.pdf (2017).
National Institute for Health and Care Excellence. Golimumab for treating non-radiographic axial spondyloarthritis. https://www.nice.org.uk/guidance/TA497 (2018)
Deodhar, A. et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration’s comments and concerns. Arthritis Rheumatol. 66, 2649–2656 (2014).
Landewe, R. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann. Rheum. Dis. 73, 39–47 (2014).
Sieper, J. Different approaches to drug approval by EMA and FDA — the example of non-radiographic axial spondyloarthritis [SP0108]. Ann. Rheum. Dis. 75, 27 (2016).
Deodhar, A. et al. A fifty-two-week, randomized, placebo-controlled trial of certolizumab pegol in nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 71, 1101–1111 (2019).
Robinson, P. C. et al. Axial spondyloarthritis: a new disease entity, not necessarily early ankylosing spondylitis. Ann. Rheum. Dis. 72, 162–164 (2013).
Akkoc, N. & Khan, M. A. Looking into the new ASAS classification criteria for axial spondyloarthritis through the other side of the glass. Curr. Rheumatol. Rep. 17, 515 (2015).
Braun, J. et al. Classification and diagnosis of axial spondyloarthritis — what is the clinically relevant difference? J. Rheumatol. 42, 31–38 (2015).
Robinson, P. C., Sengupta, R. & Siebert, S. Non-radiographic axial spondyloarthritis (nr-axSpA): advances in classification, imaging and therapy. Rheumatol. Ther. 6, 165–177 (2019).
Proft, F. & Poddubnyy, D. Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. Ther. Adv. Musculoskelet. Dis. 10, 129–139 (2018).
Renson, T. et al. High prevalence of sacroiliac bone marrow edema on MRI in post partum women: a temporary phenomenom [abstract]. Arthritis Rheumatol. 71, 597 (2019).
Lambert, R. G. et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann. Rheum. Dis. 75, 1958–1963 (2016).
Lukas, C. et al. MRI for diagnosis of axial spondyloarthritis: major advance with critical limitations ‘Not everything that glisters is gold (standard)’. RMD Open 4, e000586 (2018).
van Tubergen, A. & Weber, U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat. Rev. Rheumatol. 8, 253–261 (2012).
Bradbury, L. A. et al. Diffusion-weighted imaging is a sensitive and specific magnetic resonance sequence in the diagnosis of ankylosing spondylitis. J. Rheumatol. 45, 771–778 (2018).
Weber, U. et al. Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis Care Res. 65, 977–85 (2013).
Weber, U. et al. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res. 62, 1763–71 (2010).
Weber, U. et al. MRI of the sacroiliac joints in athletes: recognition of non-specific bone marrow oedema by semi-axial added to standard semi-coronal scans. Rheumatology 59, 1381–1390 (2020).
van der Heijde, D. et al. Spinal inflammation in the absence of sacroiliac joint inflammation on magnetic resonance imaging in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 66, 667–73 (2014).
Weber, U. et al. Does spinal MRI add incremental diagnostic value to MRI of the sacroiliac joints alone in patients with non-radiographic axial spondyloarthritis? Ann. Rheum. Dis. 74, 985–92 (2015).
Weber, U. et al. Diagnostic utility of candidate definitions for demonstrating axial spondyloarthritis on magnetic resonance imaging of the spine. Arthritis Rheumatol. 67, 924–33 (2015).
Costello, M. E. et al. Brief report: intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 67, 686–691 (2015).
Costello, M. E. et al. The intestinal microbiome in human disease and how it relates to arthritis and spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 29, 202–212 (2015).
Arnbak, B. et al. Associations between spondyloarthritis features and magnetic resonance imaging findings: a cross-sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol. 68, 892–900 (2016).
Markus, K. A. Constructs, concepts and the worlds of possibility: connecting the measurement, manipulation, and meaning of variables. Measurement 6, 54–77 (2008).
Taylor, W. & Fransen, J. Distinctions between diagnostic and classification criteria: comment on the article by Aggawal et al. Arthritis Care Res. 68, 149–150 (2015).
Dragulinescu, S. Diseases as natural kinds. Theor. Med. Bioeth. 31, 347–69 (2010).
Williams, N. E. Arthritis and nature’s joints, in Carving Nature at its joints. in Natural Kinds in Metaphysics and Science (eds Campbell, J. K, O’Rourke, M. & Slater, M. H.) (MIT Press, 2011).
Robinson, P. C. et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol. 67, 140–151 (2015).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussion of the content, writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.C.R. and W.J.T. are not members of ASAS and are not involved in the design or conduct of the CLASSIC study. P.C.R. declares that he has received research grants from Janssen, Novartis, Pfizer and UCB and has received speakers’ fees and/or acted as a consultant for AbbVie, Lilly, Gilead, Janssen, Novartis, Pfizer, Roche and UCB and received support to attend a meeting from BMS. M.A.K. is a member of ASAS and was involved in the design but not in the conduct of the CLASSIC study. M.A.K. declares that he has acted as a consultant for AbbVie, Lilly and Novartis, and has received speakers’ fees from AbbVie and Novartis. S.v.d.L. is a member of ASAS and is not involved in the design or conduct of the CLASSIC study.
Additional information
Peer review information
Nature Reviews Rheumatology thanks R. Sengupta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Robinson, P.C., van der Linden, S., Khan, M.A. et al. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol 17, 109–118 (2021). https://doi.org/10.1038/s41584-020-00552-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-00552-4
This article is cited by
-
Clinical features and fecal microbiota characteristics of patients with both ulcerative colitis and axial spondyloarthritis
BMC Gastroenterology (2024)
-
Long non-coding RNA (H19) in patients with spondyloarthritis: association with disease parameters and ultrasonographic findings
Egyptian Rheumatology and Rehabilitation (2024)
-
Prevalence of axial spondyloarthritis in Colombia: data from the National Health Registry 2017–2021
Clinical Rheumatology (2024)
-
Comparison of clinical characteristics between adult-onset and juvenile-onset non-radiographic axial spondyloarthritis in Chinese patients: results from the COCAS cohort
European Journal of Medical Research (2023)
-
The bone marrow side of axial spondyloarthritis
Nature Reviews Rheumatology (2023)