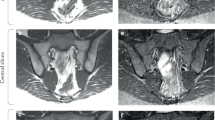
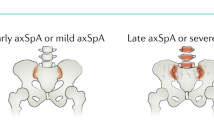
Abstract
Concerns have been raised that randomized placebo-controlled trials (RCTs) in non-radiographic axial spondyloarthritis (nr-axSpA) might be failing to identify patients that best show differences in clinical response rates between those receiving active drug and those receiving placebo therapies; in addition, some studies might even be showing spurious differences in responses to TNF and IL-17 inhibitor therapies. In particular, the most recent phase III RCTs in nr-axSpA have reported variable and generally lower response rates than observed in phase III trials of patients with ankylosing spondylitis and in trials conducted a decade ago in patients with early axSpA who were selected on the basis of axial inflammation evident on MRI scans. We argue that these observations at least partly reflect an RCT design that does not take full advantage of MRI to select patients who are responsive to therapy because the current MRI-based inclusion criteria cannot identify patients with axSpA with sufficient specificity. We propose that future studies should be designed using revised patient inclusion criteria based on expanded MRI evaluation and the application of data-driven definitions of a positive MRI for inflammatory and structural lesions typical of axSpA reported in an international multicentre analysis of MRI scans from the Assessment of SpondyloArthritis International Society (ASAS) classification cohort.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Anderson, J. J. et al. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 43, 22–29 (2000).
Wang, R. et al. Predicting probability of response to tumor necrosis factor inhibitors for individual patients with ankylosing spondylitis. JAMA Netw. Open 5, e222312 (2022).
Theander, E. et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann. Rheum. Dis. 73, 407–413 (2014).
FDA. Rheumatoid Arthritis: Developing Drug Products for Treatment. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/rheumatoid-arthritis-developing-drug-products-treatment (2013).
European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of rheumatoid arthritis. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-rheumatoid-arthritis_en.pdf (2018).
European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of psoriatic arthritis. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-psoriatic-arthritis_en.pdf (2007).
Monti, S. et al. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 1, e000057 (2015).
Maksymowych, W. P. Controversies in conventional radiography in spondyloarthritis. Best. Pract. Res. Clin. Rheumatol. 26, 839–852 (2012).
Christiansen, A. A. et al. Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J. Rheumatol. 44, 70–77 (2017).
van den Berg, R. et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol. 66, 2403–2411 (2014).
Rudwaleit, M. et al. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 52, 1000–1008 (2005).
van der Linden, S. et al. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Rudwaleit, M. et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 60, 712–727 (2009).
Rudwaleit, M. et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Rudwaleit, M. et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 68, 1520–1527 (2009).
Lambert, R. G. W. et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann. Rheum. Dis. 75, 1958–1963 (2016).
Poddubnyy, D. Radiographic evaluation of sacroiliac joints in axial spondyloarthritis–still worth performing? J. Rheumatol. 44, 1–3 (2017).
van Tubergen, A. et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann. Rheum. Dis. 62, 519–525 (2003).
Bennett, P. H. & Burch, T. A. (eds) Population Studies of the Rheumatic Diseases. 456–457 (Excerpta Medica Foundation, 1968).
Tuite, M. J. Sacroiliac joint imaging. Semin. Musculoskelet. Radiol. 12, 72–82 (2008).
van den Berg, R. et al. Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann. Rheum. Dis. 74, 2016–2021 (2015).
Maksymowych, W. P. et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann. Rheum. Dis. 78, 1550–1558 (2019).
Deodhar, A. et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration’s comments and concerns. Arthritis Rheumatol. 66, 2649–2656 (2014).
Sieper, J. et al. The Assessment of Spondyloarthritis International Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 68, ii1–ii44 (2009).
Haibel, H. et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis. Arthritis Rheumatol. 58, 1981–1991 (2008).
Song, I. H. et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomized controlled trial. Ann. Rheum. Dis. 70, 590–596 (2011).
Barkham, N. et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum. 60, 946–954 (2009).
Sieper, J. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822 (2013).
van der Heijde, D. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis. Arthritis Rheumatol. 54, 2136–2146 (2006).
Sieper, J. et al. Impact of time since diagnosis, age, and number of prior non-steroidal anti-in!ammatory drugs on response to adalimumab in patients with ankylosing spondylitis. Arthritis Rheumatol. 68, 735 (2016).
Song, I. H. et al. Similar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trial. Ann. Rheum. Dis. 72, 823–825 (2013).
Maksymowych, W. P. et al. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann. Rheum. Dis. 75, 1328–1335 (2016).
van der Heijde, D. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 52, 582–591 (2005).
Sieper, J. et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Ann. Rheum. Dis. 67, 2702–2712 (2015).
Landewé, R. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann. Rheum. Dis. 73, 39–47 (2014).
Deodhar, A. et al. A fifty-two-week, randomized, placebo-controlled trial of certolizumab pegol in nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 71, 1101–1111 (2019).
Inman, R. D. et al. Efficacy and safety of Golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 58, 3402–3412 (2008).
Deodhar, A. et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 73, 110–120 (2021).
Deodhar, A. et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 395, 53–64 (2020).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
van der Heijde, D. et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392, 2441–2451 (2018).
Maksymowych, W. P. et al. Predictors of long-term clinical response in patients with non-radiographic axial spondyloarthritis receiving certolizumab pegol. Arthritis Res. Ther. 23, 274 (2021).
de Winter, J. J. et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a metaanalysis. Arthritis Res. Ther. 18, 196 (2016).
van Hoeven, L. et al. Identifying axial spondyloarthritis in Dutch primary care patients, ages 20–45 years, with chronic low back pain. Arthritis Care. Res. 66, 446–453 (2014).
Porter, D. et al. Classification criteria: time for a rethink? Ann. Rheum. Dis. 79, 1264–1266 (2020).
De Winter, J. et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the Assessment of SpondyloArthritis International Society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 70, 1042–1048 (2018).
Weber, U. et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “background noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol. 70, 736–745 (2018).
Varkas, G. et al. Effect of mechanical stress on magnetic resonance imaging of the sacroiliac joints: assessment of military recruits by magnetic resonance imaging study. Rheumatology 57, 508–513 (2018).
Dougados, M. et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 66, 2091–2102 (2016).
Maksymowych, W. P. et al. Data-driven definitions for active and structural MRI lesions in the sacroiliac joint in spondyloarthritis and their predictive utility. Rheumatology 60, 4778–4789 (2021).
Seven, S. et al. Magnetic resonance imaging of lesions in the sacroiliac joints for differentiation of patients with axial spondyloarthritis from control subjects with or without pelvic or buttock pain: a prospective, cross-sectional study of 204 participants. Arthritis Rheumatol. 71, 2034–2046 (2019).
Latourte, A. et al. Imaging findings suggestive of axial spondyloarthritis in diffuse idiopathic skeletal hyperostosis. Arthritis Care Res. 70, 145–152 (2018).
de Hooge, M. et al. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann. Rheum. Dis. 75, 1308–1314 (2016).
Maksymowych, W. P. et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 53, 703–709 (2005).
Maksymowych, W. P. et al. Development and preliminary validation of the spondyloarthritis research Consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J. Rheumatol. 42, 79–86 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03993847 (2021).
van Tubergen, A. The changing clinical picture and epidemiology of spondyloarthritis. Nat. Rev. Rheumatol. 11, 110–118 (2015).
Author information
Authors and Affiliations
Contributions
W.P.M. researched data for this article and wrote this article. All authors provided a substantial contribution to the discussion of content and were involved in the review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
W.P.M. is Chief Medical Officer of CARE Arthritis Limited, has acted as a paid consultant/participated in advisory boards for AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer and UCB; received research and/or educational grants from AbbVie, Novartis, Pfizer and UCB; received speaker fees from AbbVie, Janssen, Novartis, Pfizer and UCB; and is a member of the ASAS-MRI Working Group. R.G.W.L. has consulted for AbbVie, CARE Arthritis, Parexel, Pfizer and UCB; and is a member of the ASAS-MRI Working Group. F.v.d.B. has received consultancy fees and/or speaker fees from AbbVie, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB Pharma. M.Ø. has received grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Merck and Novartis; has worked as a paid consultant for AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, CARE Arthritis, Celgene, Eli Lilly, Hospira, Janssen, Merck, Novartis, Novo Nordisk, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi and UCB; and received speaker fees from AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Hospira, Janssen, Merck, Novartis, Orion, Pfizer, Roche, Sandoz, Sanofi and UCB, and is a member of the ASAS-MRI Working Group. L.C. declares no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Uta Kiltz, who co-reviewed with Sabina Gall; Nurullah Akkoc; Philip Robinson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ASAS-MRI imaging electronic Case Report Form: https://www.carearthritis.com/mriportal/mrimagine/index/
Rights and permissions
About this article
Cite this article
Maksymowych, W.P., Lambert, R.G.W., Caplan, L. et al. Improving the design of RCTs in non-radiographic axial spondyloarthritis. Nat Rev Rheumatol 18, 481–489 (2022). https://doi.org/10.1038/s41584-022-00789-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00789-1