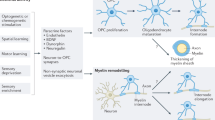
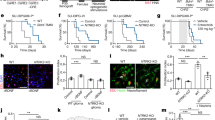
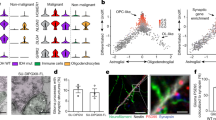
Abstract
Experience sculpts brain structure and function. Activity-dependent modulation of the myelinated infrastructure of the nervous system has emerged as a dimension of adaptive change during childhood development and in adulthood. Myelination is a richly dynamic process, with neuronal activity regulating oligodendrocyte precursor cell proliferation, oligodendrogenesis and myelin structural changes in some axonal subtypes and in some regions of the nervous system. This myelin plasticity and consequent changes to conduction velocity and circuit dynamics can powerfully influence neurological functions, including learning and memory. Conversely, disruption of the mechanisms mediating adaptive myelination can contribute to cognitive impairment. The robust effects of neuronal activity on normal oligodendroglial precursor cells, a putative cellular origin for many forms of glioma, indicates that dysregulated or ‘hijacked’ mechanisms of myelin plasticity could similarly promote growth in this devastating group of brain cancers. Indeed, neuronal activity promotes the pathogenesis of many forms of glioma in preclinical models through activity-regulated paracrine factors and direct neuron-to-glioma synapses. This synaptic integration of glioma into neural circuits is central to tumour growth and invasion. Thus, not only do neuron–oligodendroglial interactions modulate neural circuit structure and function in the healthy brain, but neuron–glioma interactions also have important roles in the pathogenesis of glial malignancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Christopherson, K. S. et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005). This work establishes that astrocytes secrete synaptogenic factors including thrombospondins.
Ullian, E. M., Harris, B. T., Wu, A., Chan, J. R. & Barres, B. A. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol. Cell Neurosci. 25, 241–251 (2004).
Eroglu, C. et al. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 (2009).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Funfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). This work demonstrates that myelinating oligodendrocytes not only modulate conduction velocity but also provide metabolic support to axons.
Huxley, A. F. & Stämpeli, R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. 108, 315–339 (1949).
Pan, Y. & Monje, M. Activity shapes neural circuit form and function: a historical perspective. J. Neurosci. 40, 944–954 (2020).
Geraghty, A. C. et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265.e8 (2019).
Knowles, J. K. et al. Maladaptive myelination promotes generalized epilepsy progression. Nat. Neurosci. 25, 596–606 (2022).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015). This work demonstrates that neuronal activity can promote the growth of high-grade gliomas.
Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019). This paper demonstrated the existence of bona fide neuron-to-glioma synapses that robustly promote tumor growth; published back-to-back with ref. 102.
Lyon, K. A. & Allen, N. J. From synapses to circuits, astrocytes regulate behavior. Front. Neural Circuits 15, 786293 (2021).
Wilton, D. K., Dissing-Olesen, L. & Stevens, B. Neuron–glia signaling in synapse elimination. Annu. Rev. Neurosci. 42, 107–127 (2019).
Khakh, B. S. & Deneen, B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207 (2019).
Nagai, J. et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron 109, 576–596 (2021).
Boullerne, A. I. The history of myelin. Exp. Neurol. 283, 431–445 (2016).
Brown, A. M., Evans, R. D., Black, J. & Ransom, B. R. Schwann cell glycogen selectively supports myelinated axon function. Ann. Neurol. 72, 406–418 (2012).
Saab, A. S., Tzvetanova, I. D. & Nave, K. A. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr. Opin. Neurobiol. 23, 1065–1072 (2013).
Zalc, B. & Colman, D. R. Origins of vertebrate success. Science 288, 271–272 (2000).
Smith, R. S. & Koles, Z. J. Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am. J. Physiol. 219, 1256–1258 (1970).
Wu, L. M. N., Williams, A., Delaney, A., Sherman, D. L. & Brophy, P. J. Increasing internodal distance in myelinated nerves accelerates nerve conduction to a flat maximum. Curr. Biol. 22, 1957–1961 (2012).
Yakovlev, P. I. in Regional Development of the Brain in Early Life Ch. 1 (ed. Minkowski, A.) 3–70 (Blackwell Scientific, 1967).
Lebel, C. et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352 (2012).
Miller, D. et al. Prolonged myelination in human neocortical evolution. Proc. Natl Acad. Sci. USA 109, 16480–16485 (2012).
Donaldson, H. A. H. &Hoke, G.W. On the areas of the axis cylinder and medullary sheath as seen in cross sections of the spinal nerves of vertebrates. J. Comp. Neurol. 15, 1–15 (1905).
Honjin, R., Sakato, S. & Yamashita, T. Electron microscopy of the mouse optic nerve: a quantitative study of the total optic nerve fibers. Arch. Histol. Jpn. 40, 321–332 (1977).
Olivares, R., Montiel, J. & Aboitiz, F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav. Evol. 57, 98–105 (2001).
Peters, A. & Sethares, C. Myelinated axons and the pyramidal cell modules in monkey primary visual cortex. J. Comp. Neurol. 365, 232–255 (1996).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014).
Lu, Q. R. et al. Common developmental requirement for olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 (2002).
Kessaris, N. et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179 (2006).
Bergles, D. & Richardson, W. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 8, a020453 (2015).
Mitew, S. et al. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276, 29–47 (2014).
Hughes, E., Kang, S., Fukaya, M. & Bergles, D. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676 (2013).
Dawson, M. R., Polito, A., Levine, J. M. & Reynolds, R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell Neurosci. 24, 476–488 (2003).
Buchanan, J. et al. Oligodendrocyte precursor cells ingest axons in the mouse neocortex. Proc. Natl Acad. Sci. USA 119, e2202580119 (2022). This work introduces the concept that OPCs can prune axons, illustrating a myelin-independent function for oligodendroglial lineage cells.
Auguste, Y. S. S. et al. Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nat. Neurosci. 25, 1273–1278 (2022). This work introduces the concept that — similar to microglia and astrocytes — OPCs can prune synapses, illustrating a myelin-independent function for oligodendroglial lineage cells.
Chung, W. S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013).
Young, K. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Tripathi, R. B. et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 21, 316–323 (2017).
Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J. & Bergles, D. E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706 (2018).
Hill, R. A., Li, A. M. & Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695 (2018).
Peters, A. & Sethares, C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cereb. Cortex 14, 995–1007 (2004).
Peters, A., Verderosa, A. & Sethares, C. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia 56, 1151–1161 (2008).
Geha, S. et al. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 20, 399–411 (2010).
Yeung, M. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Barres, B. A. & Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993).
Bergles, D. E., Roberts, J. D., Somogyi, P. & Jahr, C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000). This work demonstrates the existence of bona fide neuron-to-OPC synapses.
Lin, S. C. & Bergles, D. E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 7, 24–32 (2004).
Karadottir, R., Cavelier, P., Bergersen, L. H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005).
Stevens, B., Tanner, S. & Fields, R. D. Control of myelination by specific patterns of neural impulses. J. Neurosci. 18, 9303–9311 (1998).
Ishibashi, T. et al. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832 (2006).
Makinodan, M., Rosen, K., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Sanchez, M. M., Hearn, E. F., Do, D., Rilling, J. K. & Herndon, J. G. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 812, 38–49 (1998).
Scholz, J., Klein, M. C., Behrens, T. E. & Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009). This work shows that humans who learn a complex motor task (juggling) exhibit white matter changes consistent with myelination in relevant brain regions following training.
Schlegel, A., Rudelson, J. & Tse, P. White matter structure changes as adults learn a second language. J. Cogn. Neurosci. 24, 1664–1670 (2012).
Berendsen, S. et al. Prognostic relevance of epilepsy at presentation in glioblastoma patients. Neuro Oncol. 18, 700–706 (2016).
Lee, S., Chong, S. Y. C., Tuck, S. J., Corey, J. M. & Chan, J. R. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. protoc. 8, 771–782 (2013).
Redmond, S. A. et al. Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron 91, 824–836 (2016).
Bechler, M. E., Byrne, L. & Ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). This work demonstrates that premotor cortical projection neuron activity promotes OPC proliferation, oligodendrogenesis and myelin changes that alter motor function in an oligodendrogenesis and myelin-dependent manner.
McKenzie, I. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014). This work demonstrates that oligodendrogenesis is induced by motor learning and that motor learning in a complex wheel task depends on oligodendrogenesis.
Sampaio-Baptista, C. et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 (2013).
Mitew, S. et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. commun. 9, 306 (2018).
Steadman, P. E. et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164 e156 (2020). This work demonstrates that learning-induced oligodendrogenesis and de novo myelination is required for memory consolidation in spatial and fear learning tests, and shows that these adaptive oligodendroglial changes promote oscillatory synchrony between the hippocampus and frontal cortex.
Swire, M., Kotelevtsev, Y., Webb, D. J., Lyons, D. A. & Ffrench-Constant, C. Endothelin signalling mediates experience-dependent myelination in the CNS. eLife 8, e49493 (2019).
Yang, S. M., Michel, K., Jokhi, V., Nedivi, E. & Arlotta, P. Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370, eabd2109 (2020).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Vigano, F., Mobius, W., Gotz, M. & Dimou, L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 16, 1370–1372 (2013).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Dimou, L., Simon, C., Kirchhoff, F., Takebayashi, H. & Gotz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 28, 10434–10442 (2008).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015). This work demonstrates that synaptic vesicle release by axons regulates the number of myelin internodes that an oligodendrocyte forms and promotes myelination during development in zebrafish.
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015). This work demonstrates that myelination preferentially forms on active axons during development in zebrafish.
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R. & Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23, 487–499 (2020).
Huang, H., Zhou, F., Zhou, S. & Qiu, M. MYRF: a mysterious membrane-bound transcription factor involved in myelin development and human diseases. Neurosci. Bull. 37, 881–884 (2021).
Pajevic, S., Basser, P. & Fields, R. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147 (2014).
Noori, R. et al. Activity-dependent myelination: a glial mechanism of oscillatory self-organization in large-scale brain networks. Proc. Natl Acad. Sci. USA 117, 13227–13237 (2020).
Mount, C. W., Yalcin, B., Cunliffe-Koehler, K., Sundaresh, S. & Monje, M. Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. eLife 8, e49291 (2019).
Knowles, J. K., Batra, A., Xu, H. & Monje, M. Adaptive and maladaptive myelination in health and disease. Nat. Rev. Neurol. 18, 735–746 (2022).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 24, v1–v95 (2022).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Venteicher, A. S. et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, eaai8478 (2017).
Filbin, M. G. et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 360, 331–335 (2018).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849.e21 (2019).
Chen, J. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526 (2012).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Ryall, S. et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37, 569–583.e5 (2020).
Claus, E. B. et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg. Focus 38, E6 (2015).
Mackay, A. et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32, 520–537.e5 (2017).
Dvorak, A. V. et al. An atlas for human brain myelin content throughout the adult life span. Sci. Rep. 11, 269 (2021).
Kinney, H. C., Brody, B. A., Kloman, A. S. & Gilles, F. H. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J. Neuropathol. Exp. Neurol. 47, 217–234 (1988).
Galvao, R. P. et al. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1414389111 (2014).
Alcantara Llaguno, S. R. et al. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell 28, 429–440 (2015).
Liu, C. et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146, 209–221 (2011). This paper implicates OPCs as the cell of origin for glioblastoma.
Monje, M. et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl Acad. Sci. USA 108, 4453–4458 (2011).
Nagaraja, S. et al. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell 31, 635–652.e6 (2017).
Wang, Z. et al. Cell lineage-based stratification for glioblastoma. Cancer Cell 38, 366–379.e8 (2020).
Filbin, M. & Monje, M. Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat. Med. 25, 367–376 (2019).
Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019). Published back to back together with ref. 12, this work demonstrates the existence of bona fide neuron-to-glioma synapses that robustly promote tumour growth.
Pan, Y. et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 594, 277–282 (2021). This work shows that visual experience regulates the initiation, growth and maintenance of NF1-associated optic pathway glioma in a genetically engineered mouse model.
Toonen, J. A., Ma, Y. & Gutmann, D. H. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. 19, 808–819 (2017).
Anastasaki, C. et al. Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nat. commun. 13, 2785 (2022).
Chen, P. et al. Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature 606, 550–556 (2022).
Huang-Hobbs, E. et al. Remote neuronal activity drives glioma progression through SEMA4F. Nature 619, 844–850 (2023).
Bemben, M. A., Shipman, S. L., Nicoll, R. A. & Roche, K. W. The cellular and molecular landscape of neuroligins. Trends Neurosci. 38, 496–505 (2015).
Sudhof, T. C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Budreck, E. C. & Scheiffele, P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 26, 1738–1748 (2007).
Venkatesh, H. S. et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017).
US National Library of Medicine. ClinicalTrials.gov, h. c. g. c. s. N.
Jung, E. et al. Tweety-homolog 1 drives brain colonization of gliomas. J. Neurosci. 37, 6837–6850 (2017).
Osswald, M. et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015). This work demonstrates that glioma cells connect to each other through long processes called tumour microtubes to form a gap junction-coupled network.
Sommer, B., Kohler, M., Sprengel, R. & Seeburg, P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 (1991).
Hollmann, M., Hartley, M. & Heinemann, S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 252, 851–853 (1991).
Cull-Candy, S. G. & Farrant, M. Ca2+-permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease. J. Physiol. 599, 2655–2671 (2021).
Chen, T. J. et al. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 25, 852–861.e7 (2018).
Kougioumtzidou, E. et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6, e28080 (2017).
Taylor, K. R. et al. Glioma synapses recruit mechanisms of adaptive plasticity. Preprint at bioRxiv https://doi.org/10.1101/2021.11.04.467325 (2021).
Labrakakis, C., Patt, S., Hartmann, J. & Kettenmann, H. Functional GABAA receptors on human glioma cells. Eur. J. Neurosci. 10, 231–238 (1998).
Barron, T. et al. GABAergic neuron-to-glioma synapses in diffuse midline gliomas. Preprint at bioRxiv https://doi.org/10.1101/2022.11.08.515720 (2022).
Venkataramani, V. et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 185, 2899–2917.e31 (2022). This work shows that neuron-to-glioma synapses promote glioblastoma invasion.
Varn, F. S. et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 185, 2184–2199.e16 (2022).
Curry, R. N. et al. Glioma epileptiform activity and progression are driven by IGSF3-mediated potassium dysregulation. Neuron 111, 682–695.e9 (2023).
Sibille, J., Pannasch, U. & Rouach, N. Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J. Physiol. 592, 87–102 (2014).
Abdul Kadir, L., Stacey, M. & Barrett-Jolley, R. Emerging roles of the membrane potential: action beyond the action potential. Front. Physiol. 9, 1661 (2018).
Yang, M. & Brackenbury, W. J. Membrane potential and cancer progression. Front. Physiol. 4, 185 (2013).
LoTurco, J. J., Owens, D. F., Heath, M. J., Davis, M. B. & Kriegstein, A. R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298 (1995).
Canudas, A. M. et al. PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J. Neurosci. 24, 10343–10352 (2004).
Luk, K. C., Kennedy, T. E. & Sadikot, A. F. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J. Neurosci. 23, 2239–2250 (2003).
Luk, K. C. & Sadikot, A. F. Glutamate and regulation of proliferation in the developing mammalian telencephalon. Dev. Neurosci. 26, 218–228 (2004).
Paez-Gonzalez, P., Asrican, B., Rodriguez, E. & Kuo, C. T. Identification of distinct ChAT+ neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat. Neurosci. 17, 934–942 (2014).
Zhang, Z. et al. Activation of type 5 metabotropic glutamate receptor promotes the proliferation of rat retinal progenitor cell via activation of the PI-3-K and MAPK signaling pathways. Neuroscience 322, 138–151 (2016).
Gu, X., Olson, E. C. & Spitzer, N. C. Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 14, 6325–6335 (1994).
Weissman, T. A., Riquelme, P. A., Ivic, L., Flint, A. C. & Kriegstein, A. R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43, 647–661 (2004).
Vitali, I. et al. Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell 174, 1264–1276.e15 (2018).
Smith, R. S. et al. Sodium channel SCN3A (NaV1.3) regulation of human cerebral cortical folding and oral motor development. Neuron 99, 905–913.e7 (2018).
Smith, R. S. & Walsh, C. A. Ion channel functions in early brain development. Trends Neurosci. 43, 103–114 (2020).
Krishna, S. et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 617, 599–607 (2023). This work demonstrates that glioblastoma remodels functional neural circuitry in the human brain to augment functional connectivity with the tumour, that the synaptogenic factor TSP1 has an important role in this and that the degree of functional connectivity between glioma and the rest of the brain is strongly inversely correlated with survival.
Hausmann, D. et al. Autonomous rhythmic activity in glioma networks drives brain tumour growth. Nature 613, 179–186 (2023).
Weisbrod, D. et al. SK4 Ca2+ activated K+ channel is a critical player in cardiac pacemaker derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 110, E1685–E1694 (2013).
Buckingham, S. C. et al. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 17, 1269–1274 (2011). This work shows that glioma cells promote neuronal hyperexcitability through glutamate secretion, contributing to the common clinical phenomenon of glioma-associated seizures.
Campbell, S. L., Buckingham, S. C. & Sontheimer, H. Human glioma cells induce hyperexcitability in cortical networks. Epilepsia 53, 1360–1370 (2012).
John Lin, C. C. et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 20, 396–405 (2017).
van Breemen, M. S., Wilms, E. B. & Vecht, C. J. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 6, 421–430 (2007).
Drumm, M. R. et al. Postoperative risk of IDH-mutant glioma-associated seizures and their potential management with IDH-mutant inhibitors. J. Clin. Invest. 133, e168035 (2023).
Hatcher, A. et al. Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J. Clin. Invest. 130, 2286–2300 (2020).
Campbell, S. L. et al. GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia 63, 23–36 (2015).
Yu, K. et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578, 166–171 (2020). This work shows that glioma cells secrete synaptogenic factors such as glypican 3 to promote neuronal hyperexcitability and seizures.
Joseph, J. V. et al. TGF-β promotes microtube formation in glioblastoma through thrombospondin 1. Neuro Oncol. 24, 541–553 (2022).
Chen, C. C. L. et al. Histone H3.3G34-mutant interneuron progenitors co-opt PDGFRA for gliomagenesis. Cell 183, 1617–1633.e22 (2020).
Gibson, P. et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 468, 1095–1099 (2010).
Smith, K. S. et al. Unified rhombic lip origins of group 3 and group 4 medulloblastoma. Nature 609, 1012–1020 (2022).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Chen, Q. et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Monje, M. et al. Roadmap for the emerging field of cancer neuroscience. Cell 181, 219–222 (2020).
Winkler, F. et al. Cancer neuroscience: state of the field, emerging directions. Cell 186, 1689–1707 (2023).
Mancusi, R. & Monje, M. The neuroscience of cancer. Nature 618, 467–479 (2023).
Venkatesh, H. & Monje, M. Neuronal activity in ontogeny and oncology. Trends Cancer 3, 89–112 (2017).
Reitman, Z. J. et al. Mitogenic and progenitor gene programmes in single pilocytic astrocytoma cells. Nat. commun. 10, 3731 (2019).
Tirosh, I. et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 539, 309–313 (2016).
Liu, I. et al. The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location. Nat. Genet. 54, 1881–1894 (2022).
Beera, K. G. et al. Altered brain morphology after focal radiation reveals impact of off-target effects: implications for white matter development and neurogenesis. Neuro Oncol. 20, 788–798 (2018).
Menning, S. et al. Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain Imaging Behav. 12, 324–334 (2018).
Gibson, E. M. et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55.e13 (2019).
Fernandez-Castaneda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185, 2452–2468.e16 (2022).
Monje, M. & Iwasaki, A. The neurobiology of long COVID. Neuron 110, 3484–3496 (2022).
Verhaak, R. G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Acknowledgements
The authors thank M. Filbin for helpful input on Table 1. This work was supported by grants from Cancer Research UK (to M.M.), ChadTough Defeat DIPG (to M.M.), National Institute of Neurological Disorders and Stroke (R01NS092597 to M.M.), National Institutes of Health (NIH) Director’s Pioneer Award (DP1NS111132 to M.M.), National Cancer Institute (P50CA165962, R01CA258384, U19CA264504 to M.M.), Damon Runyon Cancer Research Foundation (to K.R.T.), Stanford Maternal and Child Health Research Institute (to K.R.T.), Gatsby Charitable Foundation (Gatsby Initiative in Brain Development and Psychiatry to M.M.), HHMI Emerging Pathogens Initiative (EPI), Oscar’s Kids Foundation (to M.M.), McKenna Claire Foundation (to M.M.), Virginia and D. K. Ludwig Fund for Cancer Research (to M.M.), Oligo Nation (to M.M.), Waxman Family Research Fund (to M.M.) and Will Irwin Research Fund (to M.M.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.M. is on the SAB for TippingPoint Biosciences, and her family holds equity in MapLight Therapeutics.
Peer review
Peer review information
Nature Reviews Neuroscience thanks William Weiss, who co-reviewed with Ekin Guney; Klaus-Armin Nave; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taylor, K.R., Monje, M. Neuron–oligodendroglial interactions in health and malignant disease. Nat. Rev. Neurosci. 24, 733–746 (2023). https://doi.org/10.1038/s41583-023-00744-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00744-3