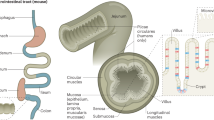
Abstract
The intestine, like the liver and kidney, in various vertebrates and humans is able to carry out gluconeogenesis and release glucose into the blood. In the fed post-absorptive state, intestinal glucose is sensed by the gastrointestinal nervous system. The latter initiates a signal to the brain regions controlling energy homeostasis and stress-related behaviour. Intestinal gluconeogenesis (IGN) is activated by several complementary mechanisms, in particular nutritional situations (for example, when food is enriched in protein or fermentable fibre and after gastric bypass surgery in obesity). In these situations, IGN has several metabolic and behavioural benefits. As IGN is activated by nutrients capable of fuelling systemic gluconeogenesis, IGN could be a signal to the brain that food previously ingested is suitable for maintaining plasma glucose for a while. This process might account for the benefits observed. Finally, in this Perspective, we discuss how the benefits of IGN in fasting and fed states could explain why IGN emerged and was maintained in vertebrates by natural selection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Accession numbers of the amino acid sequences used for Fig. 4 are provided as an Excel spreadsheet (Supplementary Table 1); each row corresponds to a protein-coding gene. Protein-coding genes present in the phylogenetic tree of Fig. 4b are indicated in red. The sequence alignment corresponding to the TreeFam family TF324388 is provided as a text file (Supplementary Table 2). These data were freely obtained from the TreeFam v9 database hosted at the European Bioinformatics Institute (EBI).
References
Soty, M., Gautier-Stein, A., Rajas, F. & Mithieux, G. Gut-brain glucose signaling in energy homeostasis. Cell Metab. 25, 1231–1242 (2017).
Bernard, C. Nouvelle fonction du foie considéré comme organe producteur de matière sucrée chez l’homme et les animaux (J.-B. Baillie, 1853).
Krebs, H. A. Renal gluconeogenesis. Adv. Enzym. Regul. 1, 385–400 (1963).
Rajas, F., Bruni, N., Montano, S., Zitoun, C. & Mithieux, G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 117, 132–139 (1999).
Croset, M. et al. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes 50, 740–746 (2001).
Battezzati, A. et al. Nonhepatic glucose production in humans. Am. J. Physiol. Endocrinol. Metab. 286, E129–E135 (2004).
Hayes, M. T., Foo, J., Besic, V., Tychinskaya, Y. & Stubbs, R. S. Is intestinal gluconeogenesis a key factor in the early changes in glucose homeostasis following gastric bypass? Obes. Surg. 21, 759–762 (2011).
Mithieux, G. Comment about intestinal gluconeogenesis after gastric bypass in human in relation with the paper by Hayes et al., Obes. Surg. 2011. Obes. Surg. 22, 1920–1922 (2012).
Gerich, J. E., Meyer, C., Woerle, H. J. & Stumvoll, M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24, 382–391 (2001).
Pillot, B., Soty, M., Gautier-Stein, A., Zitoun, C. & Mithieux, G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology 150, 616–624 (2009).
Owen, O. E., Felig, P., Morgan, A. P., Wahren, J. & Cahill, G. F. Liver and kidney metabolism during prolonged starvation. J. Clin. Invest. 48, 574–583 (1969).
Mutel, E. et al. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes 60, 3121–3131 (2011).
Russek, M. Participation of hepatic glucoreceptors in the control of intake of food. Nature 197, 79–80 (1963).
Tordoff, M. G. & Friedman, M. I. Hepatic portal glucose infusions decrease food intake and increase food preference. Am. J. Physiol. 251, R192–R196 (1986).
Niijima, A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J. Physiol. 332, 315–323 (1982).
Fournel, A. et al. Glucosensing in the gastrointestinal tract: Impact on glucose metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G645–G658 (2016).
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Duca, F. A., Waise, T. M. Z., Peppler, W. T. & Lam, T. K. T. The metabolic impact of small intestinal nutrient sensing. Nat. Commun. 12, 903 (2021).
Booth, D. A., Chase, A. & Campbell, A. T. Relative effectiveness of protein in the late stages of appetite suppression in man. Physiol. Behav. 5, 1299–1302 (1970).
Rolls, B. J., Hetherington, M. & Burley, V. J. The specificity of satiety: the influence of foods of different macronutrient content on the development of satiety. Physiol. Behav. 43, 145–153 (1988).
Mithieux, G. et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2, 321–329 (2005).
Lee, V. H. Membrane transporters. Eur. J. Pharm. Sci. 11, S41–S50 (2000).
Duraffourd, C. et al. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell 150, 377–388 (2012).
Penhoat, A. et al. Protein-induced satiety is abolished in the absence of intestinal gluconeogenesis. Physiol. Behav. 105, 89–93 (2011).
Ray, T. K. et al. Long-term effects of dietary fiber on glucose tolerance and gastric emptying in noninsulin-dependent diabetic patients. Am. J. Clin. Nutr. 37, 376–381 (1983).
Mendeloff, A. I. Dietary fiber and human health. N. Engl. J. Med. 297, 811–814 (1977).
Mithieux, G., Gautier-Stein, A., Rajas, F. & Zitoun, C. Contribution of intestine and kidney to glucose fluxes in different nutritional states in rat. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 143, 195–200 (2006).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014).
De Vadder, F., Plessier, F., Gautier-Stein, A. & Mithieux, G. Vasoactive intestinal peptide is a local mediator in a gut-brain neural axis activating intestinal gluconeogenesis. Neurogastroenterol. Motil. 27, 443–448 (2015).
Flint, H. J., Scott, K. P., Louis, P. & Duncan, S. H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
De Vadder, F. et al. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 24, 151–157 (2016).
Wang, K. et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5 (2019).
Minassian, C., Ajzannay, A., Riou, J. P. & Mithieux, G. Investigation of the mechanism of glycogen rebound in the liver of 72-hour fasted rats. J. Biol. Chem. 269, 16585–16588 (1994).
Mithieux, G., Daniele, N., Payrastre, B. & Zitoun, C. Liver microsomal glucose-6-phosphatase is competitively inhibited by the lipid products of phosphatidylinositol 3-kinase. J. Biol. Chem. 273, 17–19 (1998).
Daniele, N. et al. Phosphatidylinositol 3-kinase translocates onto liver endoplasmic reticulum and may account for the inhibition of glucose-6-phosphatase during refeeding. J. Biol. Chem. 274, 3597–3601 (1999).
Mithieux, G. The gut microbiota: stable bioreactor of variable composition? Trends Endocrinol. Metab. 33, 443–446 (2022).
Pories, W. J. Diabetes: the evolution of a new paradigm. Ann. Surg. 239, 12–13 (2004).
Barataud, A. et al. Metabolic benefits of gastric bypass surgery in the mouse: the role of fecal losses. Mol. Metab. 31, 14–23 (2020).
Troy, S. et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 8, 201–211 (2008).
Kim, M., Son, Y. G., Kang, Y. N., Ha, T. K. & Ha, E. Changes in glucose transporters, gluconeogenesis, and circadian clock after duodenal–jejunal bypass surgery. Obes. Surg. 25, 635–641 (2015).
Sun, D. et al. Duodenal-jejunal bypass surgery up-regulates the expression of the hepatic insulin signaling proteins and the key regulatory enzymes of intestinal gluconeogenesis in diabetic Goto-Kakizaki rats. Obes. Surg. 23, 1734–1742 (2013).
Yan, Y. et al. Roux-en-Y gastric bypass surgery suppresses hepatic gluconeogenesis and increases intestinal gluconeogenesis in a T2DM rat model. Obes. Surg. 26, 2683–2690 (2016).
Gutierrez-Repiso, C. et al. Jejunal gluconeogenesis associated with insulin resistance level and its evolution after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 13, 623–630 (2017).
Jean, C. et al. Metabolic evidence for adaptation to a high protein diet in rats. J. Nutr. 131, 91–98 (2001).
Sjöström, L. et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 351, 2683–2693 (2004).
Vily-Petit, J. et al. Intestinal gluconeogenesis regulates brown and white adipose tissues functions in mice. Preprint at bioRxiv https://doi.org/10.1101/2021.10.25.465675 (2021).
Vily-Petit, J. et al. Intestinal gluconeogenesis prevents obesity-linked liver steatosis and non-alcoholic fatty liver disease. Gut 69, 2193–2202 (2020).
Soty, M. et al. A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Mol. Metab. 4, 106–117 (2015).
Parlati, L., Régnier, M., Guillou, H. & Postic, C. New targets for NAFLD. JHEP Rep. 3, 100346 (2021).
Delaere, F. et al. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol. Metab. 2, 47–53 (2012).
Delaere, F., Akaoka, H., De Vadder, F., Duchampt, A. & Mithieux, G. Portal glucose influences the sensory, cortical and reward systems in rats. Eur. J. Neurosci. 38, 3476–3486 (2013).
Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 58, 221–232 (2015).
Diez-Sampedro, A. et al. A glucose sensor hiding in a family of transporters. Proc. Natl Acad. Sci. 100, 11753–11758 (2003).
Soták, M., Marks, J. & Unwin, R. J. Putative tissue location and function of the SLC5 family member SGLT3: developments in SGLT3 biology. Exp. Physiol. 102, 5–13 (2017).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013).
Soty, M. et al. Calcitonin gene-related peptide-induced phosphorylation of STAT3 in arcuate neurons is a link in the metabolic benefits of portal glucose. Neuroendocrinology 111, 555–567 (2021).
Nogueiras, R., López, M. & Diéguez, C. Regulation of lipid metabolism by energy availability: a role for the central nervous system. Obes. Rev. 11, 185–201 (2010).
Lee, S. B. et al. Intermittent restraint-induced sympathetic activation attenuates hepatic steatosis and inflammation in a high-fat diet-fed mouse model. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G811–G823 (2019).
Morrison, S. F., Madden, C. J. & Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756 (2014).
Bachman, E. S. et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Berland, C., Small, D. M., Luquet, S. & Gangarossa, G. Dietary lipids as regulators of reward processes: multimodal integration matters. Trends Endocrinol. Metab. 32, 693–705 (2021).
Sinet, F. et al. Dietary fibers and proteins modulate behavior via the activation of intestinal gluconeogenesis. Neuroendocrinology 111, 1249–1265 (2021).
Pariante, C. M. & Miller, A. H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry 49, 391–404 (2001).
Zhu, L.-J. et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity. PLoS ONE 9, e97689 (2014).
Zemdegs, J. et al. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br. J. Pharmacol. 173, 2095–2110 (2016).
Spencer, S. J., Korosi, A., Layé, S., Shukitt-Hale, B. & Barrientos, R. M. Food for thought: how nutrition impacts cognition and emotion. NPJ Sci. Food 1, 7 (2017).
Wolfe, A. R. et al. Dietary protein and protein-rich food in relation to severely depressed mood: a 10 year follow-up of a national cohort. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 232–238 (2011).
Hutton, J. C. & O’Brien, R. M. Glucose-6-phosphatase catalytic subunit gene family. J. Biol. Chem. 284, 29241–29245 (2009).
Hübscher, G., West, G. R. & Brindley, D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem. J. 97, 629–642 (1965).
Shittu, S. T., Alada, A. R. A. & Oyebola, D. D. O. Metabolic fate of the glucose taken up by the intestine during induced hyperglycaemia in dogs. Niger. J. Physiol. Sci. 33, 37–49 (2018).
Lohrenz, A.-K. et al. Glucose transporters and enzymes related to glucose synthesis in small intestinal mucosa of mid-lactation dairy cows fed 2 levels of starch. J. Dairy. Sci. 94, 4546–4555 (2011).
Tournaire, D., Bastide, P. & Dastugue, G. Enzymatic activities in the small intestine of the lamb. (Acid and alkaline phosphatases, glucose-6-phosphatase, alpha-D-methylglucosidase, invertase and lactase). C. R. Seances Soc. Biol. Fil. 160, 1597–1600 (1966).
Radecki, S. V., Ku, P. K., Bennink, M. R., Yokoyama, M. T. & Miller, E. R. Effect of dietary copper on intestinal mucosa enzyme activity, morphology, and turnover rates in weanling pigs. J. Anim. Sci. 70, 1424–1431 (1992).
Bismut, H., Hers, H. G. & Van Schaftingen, E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur. J. Biochem. 213, 721–726 (1993).
Anderson, J. W. & Rosendall, A. F. Gluconeogenesis in jejunal mucosa of guinea pig. Biochim. Biophys. Acta 304, 384–388 (1973).
Palmer, M. F. & Rolls, B. A. The activities of some metabolic enzymes in the intestines of germ-free and conventional chicks. Br. J. Nutr. 50, 783–790 (1983).
Su, J. et al. The characteristics of glucose homoeostasis in grass carp and Chinese longsnout catfish after oral starch administration: a comparative study between herbivorous and carnivorous species of fish. Br. J. Nutr. 123, 627–641 (2020).
Chen, Y.-J. et al. Simultaneous stimulation of glycolysis and gluconeogenesis by feeding in the anterior intestine of the omnivorous GIFT tilapia, Oreochromis niloticus. Biol. Open 6, 818–824 (2017).
Kirchner, S., Seixas, P., Kaushik, S. & Panserat, S. Effects of low protein intake on extra-hepatic gluconeogenic enzyme expression and peripheral glucose phosphorylation in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 140, 333–340 (2005).
Penhoat, A., Fayard, L., Stefanutti, A., Mithieux, G. & Rajas, F. Intestinal gluconeogenesis is crucial to maintain a physiological fasting glycemia in the absence of hepatic glucose production in mice. Metabolism 63, 104–111 (2014).
Hartmann, F. & Plauth, M. Intestinal glutamine metabolism. Metabolism 38, 18–24 (1989).
Windmueller, H. G. & Spaeth, A. E. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J. Biol. Chem. 253, 69–76 (1978).
Watford, M. Glutamine metabolism in rat small intestine: synthesis of three-carbon products in isolated enterocytes. Biochim. Biophys. Acta 1200, 73–78 (1994).
Mithieux, G., Rajas, F. & Gautier-Stein, A. A novel role for glucose 6-phosphatase in the small intestine in the control of glucose homeostasis. J. Biol. Chem. 279, 44231–44234 (2004).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455 (2020).
Geiselman, P. J. & Novin, D. The role of carbohydrates in appetite, hunger and obesity. Appetite 3, 203–223 (1982).
Little, T. J. & Feinle-Bisset, C. Effects of dietary fat on appetite and energy intake in health and obesity–oral and gastrointestinal sensory contributions. Physiol. Behav. 104, 613–620 (2011).
Schwartz, M. W. & Morton, G. J. Obesity: keeping hunger at bay. Nature 418, 595–597 (2002).
Eaton, S. B. & Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 312, 283–289 (1985).
Ben-Dor, M., Sirtoli, R. & Barkai, R. The evolution of the human trophic level during the Pleistocene. Am. J. Phys. Anthropol. 175, 27–56 (2021).
Mithieux, G., Rajas, F. & Zitoun, C. Glucose utilization is suppressed in the gut of insulin-resistant high fat-fed rats and is restored by metformin. Biochem. Pharmacol. 72, 1757–1762 (2006).
Andreelli, F. et al. Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology 147, 2432–2441 (2006).
Flak, J. N. & Myers, M. G. Minireview: CNS mechanisms of leptin action. Mol. Endocrinol. 30, 3–12 (2016).
Enriori, P. J., Sinnayah, P., Simonds, S. E., Garcia Rudaz, C. & Cowley, M. A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 31, 12189–12197 (2011).
Liu, J., Perez, S. M., Zhang, W., Lodge, D. J. & Lu, X.-Y. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol. Psychiatry 16, 1024–1038 (2011).
Jacobs, B. L. Adult brain neurogenesis and depression. Brain Behav. Immun. 16, 602–609 (2002).
Garza, J. C., Guo, M., Zhang, W. & Lu, X.-Y. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3β/β-catenin signaling. Mol. Psychiatry 17, 790–808 (2012).
Londraville, R. L., Prokop, J. W., Duff, R. J., Liu, Q. & Tuttle, M. On the molecular evolution of leptin, leptin receptor, and endospanin. Front. Endocrinol. 8, 58 (2017).
Ricardo-Silgado, M. L., McRae, A. & Acosta, A. Role of enteroendocrine hormones in appetite and glycemia. Obes. Med. 23, 100332 (2021).
Kim, Y.-K., Kim, O. Y. & Song, J. Alleviation of depression by glucagon-like peptide 1 through the regulation of neuroinflammation, neurotransmitters, neurogenesis, and synaptic function. Front. Pharmacol. 11, 1270 (2020).
Crespi, F. On the role of cholecystokinin (CCK) in fear and anxiety: a review and research proposal. J. Hum. Psychol. https://doi.org/10.14302/issn.2644-1101.jhp-19-2766 (2019).
Ruan, J. et al. TreeFam: 2008 update. Nucleic Acids Res. 36, D735–D740 (2008).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Acknowledgements
The authors thank their team and the countless number of past members and collaborators, without whom the work analysed herein would not have been possible, and more particularly F. Rajas, J. Vily-Petit and E. Van Obberghen for critical reading and help in the preparation of the manuscript, and G. Escarguel, V. Balter and Y. Voituron for help in relation to the evolution of human nutrition. The authors are also grateful to the University Claude Bernard Lyon 1 and INSERM for hosting and funding their laboratory and the INRAe and the CNRS for funding their salary.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Petia Kovatcheva-Datchary, Ruben Nogueiras and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gautier-Stein, A., Mithieux, G. Intestinal gluconeogenesis: metabolic benefits make sense in the light of evolution. Nat Rev Gastroenterol Hepatol 20, 183–194 (2023). https://doi.org/10.1038/s41575-022-00707-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00707-6
This article is cited by
-
Breastfeeding is associated with enhanced intestinal gluconeogenesis in infants
BMC Medicine (2024)
-
Paneth cell-derived iNOS is required to maintain homeostasis in the intestinal stem cell niche
Journal of Translational Medicine (2023)
-
Metabolic Messengers: glucagon
Nature Metabolism (2023)