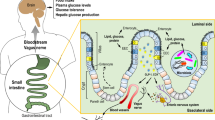
Abstract
The small intestine displays marked anatomical and functional plasticity that includes adaptive alterations in adult gut morphology, enteroendocrine cell profile and their hormone secretion, as well as nutrient utilization and storage. In this Perspective, we examine how shifts in dietary and environmental conditions bring about changes in gut size, and describe how the intestine adapts to changes in internal state, bowel resection and gastric bypass surgery. We highlight the critical importance of these intestinal remodelling processes in maintaining energy balance of the organism, and in protecting the metabolism of other organs. The intestinal resizing is supported by changes in the microbiota composition, and by activation of carbohydrate and fatty acid metabolism, which govern the intestinal stem cell proliferation, intestinal cell fate, as well as survivability of differentiated epithelial cells. The discovery that intestinal remodelling is part of the normal physiological adaptation to various triggers, and the potential for harnessing the reversible gut plasticity, in our view, holds extraordinary promise for developing therapeutic approaches against metabolic and inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hounnou, G., Destrieux, C., Desme, J., Bertrand, P. & Velut, S. Anatomical study of the length of the human intestine. Surg. Radiol. Anat. 24, 290–294 (2002).
Guzman, I. J., Fitch, L. L., Varco, R. L. & Buchwald, H. Small bowel length in hyperlipidemia and massive obesity. Am. J. Clin. Nutr. 30, 1006–1008 (1977).
Hosseinpour, M. & Behdad, A. Evaluation of small bowel measurement in alive patients. Surg. Radiol. Anat. 30, 653–655 (2008).
Bekheit, M., Ibrahim, M. Y., Tobar, W., Galal, I. & Elward, A. S. Correlation between the total small bowel length and anthropometric measures in living humans: cross-sectional study. Obes. Surg. 30, 681–686 (2020).
Helander, H. F. & Fandriks, L. Surface area of the digestive tract—revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Miguel-Aliaga, I., Jasper, H. & Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics 210, 357–396 (2018).
Ariyapala, I. S. et al. Identification of split-GAL4 drivers and enhancers that allow regional cell type manipulations of the Drosophila melanogaster intestine. Genetics 216, 891–903 (2020).
Lim, S. Y. et al. Identification and characterization of GAL4 drivers that mark distinct cell types and regions in the Drosophila adult gut. J. Neurogenet. 35, 33–44 (2021).
van Rheenen, J. & Bruens, L. Cellular protection mechanisms that minimise accumulation of mutations in intestinal tissue. Swiss Med. Wkly 147, w14539 (2017).
Baker, A. M. et al. Crypt fusion as a homeostatic mechanism in the human colon. Gut 68, 1986–1993 (2019).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Lopez-Garcia, C., Klein, A. M., Simons, B. D. & Winton, D. J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 (2010).
Sei, Y., Feng, J., Chow, C. C. & Wank, S. A. Asymmetric cell division-dominant neutral drift model for normal intestinal stem cell homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G64–G74 (2019).
Joly, A. & Rousset, R. Tissue adaptation to environmental cues by symmetric and asymmetric division modes of intestinal stem cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21176362 (2020).
Noah, T. K., Donahue, B. & Shroyer, N. F. Intestinal development and differentiation. Exp. Cell. Res. 317, 2702–2710 (2011).
Patankar, J. V. & Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 17, 543–556 (2020).
Krndija, D. et al. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365, 705–710 (2019).
Jones, D. E. & Bevins, C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267, 23216–23225 (1992).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Corr, S. C., Gahan, C. C. & Hill, C. M cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 52, 2–12 (2008).
Wozniak, D., Cichy, W., Przyslawski, J. & Drzymala-Czyz, S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv. Med. Sci. 66, 284–292 (2021).
Gribble, F. M. & Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 15, 226–237 (2019).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Azkanaz, M. et al. Retrograde movements determine effective stem cell numbers in the intestine. Nature 607, 548–554 (2022).
Beumer, J. & Clevers, H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 143, 3639–3649 (2016).
Ayyaz, A. et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125 (2019).
van Es, J. H. et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104 (2012).
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Tetteh, P. W. et al. Replacement of lost lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18, 203–213 (2016).
Marianes, A. & Spradling, A. C. Physiological and stem cell compartmentalization within the Drosophila midgut. Elife 2, e00886 (2013).
Kraiczy, J. et al. DNA methylation defines regional identity of human intestinal epithelial organoids and undergoes dynamic changes during development. Gut 68, 49–61 (2019).
Middendorp, S. et al. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells 32, 1083–1091 (2014).
Harnik, Y. et al. Spatial discordances between mRNAs and proteins in the intestinal epithelium. Nat. Metab. 3, 1680–1693 (2021).
Moor, A. E. et al. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell 175, 1156–1167 (2018).
Yang, H., Wang, X., Xiong, X. & Yin, Y. Energy metabolism in intestinal epithelial cells during maturation along the crypt–villus axis. Sci. Rep. 6, 31917 (2016).
Van Der Schoor, S. R. et al. The high metabolic cost of a functional gut. Gastroenterology 123, 1931–1940 (2002).
Tome, D. The roles of dietary glutamate in the intestine. Ann. Nutr. Metab. 73, 15–20 (2018).
Bahar Halpern, K. et al. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat. Commun. 11, 1936 (2020).
Tappenden, K. A. Intestinal adaptation following resection. JPEN J. Parenter. Enteral Nutr. 38, 23S–31S (2014).
Weale, A. R., Edwards, A. G., Bailey, M. & Lear, P. A. Intestinal adaptation after massive intestinal resection. Postgrad. Med. J. 81, 178–184 (2005).
Burrin, D. G., Ferrell, C. L., Britton, R. A. & Bauer, M. Level of nutrition and visceral organ size and metabolic activity in sheep. Br. J. Nutr. 64, 439–448 (1990).
Mao, J. et al. Overnutrition stimulates intestinal epithelium proliferation through beta-catenin signaling in obese mice. Diabetes 62, 3736–3746 (2013).
Stojanovic, O. et al. Dietary excess regulates absorption and surface of gut epithelium through intestinal PPARα. Nat. Commun. https://doi.org/10.1038/s41467-021-27133-7 (2021).
Chevalier, C. et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 163, 1360–1374 (2015).
Mercado-Perez, A. & Beyder, A. Gut feelings: mechanosensing in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-021-00561-y (2022).
Yang, Q. et al. Cell fate coordinates mechano-osmotic forces in intestinal crypt formation. Nat. Cell Biol. 23, 733–744 (2021).
Perez-Gonzalez, C. et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 23, 745–757 (2021).
O’Brien, L. E., Soliman, S. S., Li, X. & Bilder, D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 147, 603–614 (2011).
Li, Q. et al. Ingestion of food particles regulates the mechanosensing Misshapen–Yorkie pathway in Drosophila intestinal growth. Dev. Cell 45, 433–449 (2018).
Shaw, R. L. et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137, 4147–4158 (2010).
He, L., Si, G., Huang, J., Samuel, A. D. T. & Perrimon, N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 555, 103–106 (2018).
Spiljar, M. et al. Cold exposure protects from neuroinflammation through immunologic reprogramming. Cell Metab. 33, 2231–2246 (2021).
Liao, W. H., Henneberg, M. & Langhans, W. Immunity-based evolutionary interpretation of diet-induced thermogenesis. Cell Metab. 23, 971–979 (2016).
Toloza, E. M., Lam, M. & Diamond, J. Nutrient extraction by cold-exposed mice: a test of digestive safety margins. Am. J. Physiol. 261, G608–G620 (1991).
Chevalier, C. et al. Warmth prevents bone loss through the gut microbiota. Cell Metab. 32, 575–590 (2020).
Speakman, J. R. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 375–398 (2008).
Hammond, K. A. Adaptation of the maternal intestine during lactation. J. Mammary Gland Biol. Neoplasia 2, 243–252 (1997).
Weaver, L. T., Austin, S. & Cole, T. J. Small intestinal length: a factor essential for gut adaptation. Gut 32, 1321–1323 (1991).
Koren, O. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012).
Reiff, T. et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. Elife 4, e06930 (2015).
White, M. A., Bonfini, A., Wolfner, M. F. & Buchon, N. Drosophila melanogaster sex peptide regulates mated female midgut morphology and physiology. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2018112118 (2021).
Hadjieconomou, D. et al. Enteric neurons increase maternal food intake during reproduction. Nature 587, 455–459 (2020).
Le Gall, M. et al. Intestinal plasticity in response to nutrition and gastrointestinal surgery. Nutr. Rev. 77, 129–143 (2019).
Le Beyec, J., Billiauws, L., Bado, A., Joly, F. & Le Gall, M. Short bowel syndrome: a paradigm for intestinal adaptation to nutrition? Annu. Rev. Nutr. 40, 299–321 (2020).
Weser, E. Nutritional aspects of malabsorption. Short gut adaptation. Am. J. Med. 67, 1014–1020 (1979).
Booth, C. C., Evans, K. T., Menzies, T. & Street, D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br. J. Surg. 46, 403–410 (1959).
Jeppesen, P. B. & Mortensen, P. B. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut 43, 478–483 (1998).
Drucker, D. J., Erlich, P., Asa, S. L. & Brubaker, P. L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl Acad. Sci. USA 93, 7911–7916 (1996).
Brubaker, P. L. Glucagon-like peptide 2 and the regulation of intestinal growth and function. Compr. Physiol. 8, 1185–1210 (2018).
Au, A., Gupta, A., Schembri, P. & Cheeseman, C. I. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem. J. 367, 247–254 (2002).
Cheeseman, C. I. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am. J. Physiol. 273, R1965–R1971 (1997).
Camastra, S., Palumbo, M. & Santini, F. Nutrients handling after bariatric surgery, the role of gastrointestinal adaptation. Eat. Weight Disord. 27, 449–461 (2022).
Larraufie, P. et al. Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 26, e1396 (2019).
Saeidi, N. et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341, 406–410 (2013).
Troy, S. et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 8, 201–211 (2008).
McCauley, H. A. et al. Enteroendocrine cells couple nutrient sensing to nutrient absorption by regulating ion transport. Nat. Commun. 11, 4791 (2020).
Albaugh, V. L. et al. Regulation of body weight: lessons learned from bariatric surgery. Mol. Metab. https://doi.org/10.1016/j.molmet.2022.101517 (2022).
Ahmad, N. N., Pfalzer, A. & Kaplan, L. M. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int. J. Obes. 37, 1553–1559 (2013).
Albaugh, V. L. et al. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab. 100, E1225–E1233 (2015).
Calderon, G. et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EbioMedicine 55, 102759 (2020).
Li, K. et al. Farnesoid X receptor contributes to body weight-independent improvements in glycemic control after Roux-en-Y gastric bypass surgery in diet-induced obese mice. Mol. Metab. 37, 100980 (2020).
McGavigan, A. K. et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66, 226–234 (2017).
Hatoum, I. J. et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 97, E1023–E1031 (2012).
Faramia, J. et al. IGFBP-2 partly mediates the early metabolic improvements caused by bariatric surgery. Cell Rep. Med. 2, 100248 (2021).
Zhou, W., Rowitz, B. M. & Dailey, M. J. Insulin/IGF-1 enhances intestinal epithelial crypt proliferation through PI3K/Akt, and not ERK signaling in obese humans. Exp. Biol. Med. 243, 911–916 (2018).
Hao, Z. et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J. Obes. 39, 798–805 (2015).
Mokadem, M., Zechner, J. F., Uchida, A. & Aguirre, V. Leptin is required for glucose homeostasis after Roux-en-Y gastric bypass in mice. PLoS ONE 10, e0139960 (2015).
Sukhotnik, I. et al. Leptin affects intestinal epithelial cell turnover in correlation with leptin receptor expression along the villus–crypt axis after massive small bowel resection in a rat. Pediatr. Res 66, 648–653 (2009).
Leigh, S. C., Nguyen-Phuc, B. Q. & German, D. P. The effects of protein and fiber content on gut structure and function in zebrafish (Danio rerio). J. Comp. Physiol. B 188, 237–253 (2018).
McCullogh, J. S., Ratcliffe, B., Mandir, N., Carr, K. E. & Goodlad, R. A. Dietary fibre and intestinal microflora: effects on intestinal morphometry and crypt branching. Gut 42, 799–806 (1998).
Dalby, M. J., Ross, A. W., Walker, A. W. & Morgan, P. J. Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep. 21, 1521–1533 (2017).
Hunt, J. E., Hartmann, B., Schoonjans, K., Holst, J. J. & Kissow, H. Dietary fiber is essential to maintain intestinal size, L cell secretion, and intestinal integrity in mice. Front Endocrinol. 12, 640602 (2021).
Mihaylova, M. M. et al. Fasting activates fatty acid oxidation to inhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22, 769–778 (2018).
Yilmaz, O. H. et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495 (2012).
Rock, S. A. et al. Neurotensin regulates proliferation and stem cell function in the small intestine in a nutrient-dependent manner. Cell Mol. Gastroenterol. Hepatol. 13, 501–516 (2022).
Stine, R. R. et al. PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell 25, 830–845 (2019).
Goncalves, M. D. et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349 (2019).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Scoaris, C. R. et al. Effects of cafeteria diet on the jejunum in sedentary and physically trained rats. Nutrition 26, 312–320 (2010).
Petit, V. et al. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J. Lipid Res. 48, 278–287 (2007).
Xie, Y. et al. Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol. Med. Rep. 21, 1133–1144 (2020).
Cheng, C. W. et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 178, 1115–1131 (2019).
Stadler, J. et al. Effect of high fat consumption on cell proliferation activity of colorectal mucosa and on soluble faecal bile acids. Gut 29, 1326–1331 (1988).
Mah, A. T., Van Landeghem, L., Gavin, H. E., Magness, S. T. & Lund, P. K. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 155, 3302–3314 (2014).
Takase, S. & Goda, T. Effects of medium-chain triglycerides on brush border membrane-bound enzyme activity in rat small intestine. J. Nutr. 120, 969–976 (1990).
Chwen, L. T., Foo, H. L., Thanh, N. T. & Choe, D. W. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian-Australas. J. Anim. Sci. 26, 700–704 (2013).
Wang, B. et al. Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell 22, 206–220 (2018).
Aliluev, A. et al. Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat. Metab. 3, 1202–1216 (2021).
Taylor, S. R. et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature https://doi.org/10.1038/s41586-021-03827-2 (2021).
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011).
Redhai, S. et al. An intestinal zinc sensor regulates food intake and developmental growth. Nature 580, 263–268 (2020).
Obata, F. et al. Nutritional control of stem cell division through S-adenosylmethionine in Drosophila intestine. Dev. Cell 44, 741–751 (2018).
Kim, B. et al. Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit. Nature 593, 570–574 (2021).
Tan, B. L. & Norhaizan, M. E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients https://doi.org/10.3390/nu11112579 (2019).
Zhou, D., Shao, L. & Spitz, D. R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res 122, 1–67 (2014).
Gulhane, M. et al. Hig-fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 6, 28990 (2016).
Hamilton, M. K., Boudry, G., Lemay, D. G. & Raybould, H. E. Changes in intestinal barrier function and gut microbiota in high-fat-diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G840–G851 (2015).
Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A. & Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv. Nutr. 11, 77–91 (2020).
Flanagan, D. J., Austin, C. R., Vincan, E. & Phesse, T. J. Wnt signalling in gastrointestinal epithelial stem cells. Genes https://doi.org/10.3390/genes9040178 (2018).
Mah, A. T., Yan, K. S. & Kuo, C. J. Wnt pathway regulation of intestinal stem cells. J. Physiol. 594, 4837–4847 (2016).
Degirmenci, B., Valenta, T., Dimitrieva, S., Hausmann, G. & Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558, 449–453 (2018).
Maimets, M. et al. Mesenchymal–epithelial cross-talk shapes intestinal regionalisation via Wnt and Shh signalling. Nat. Commun. 13, 715 (2022).
Goga, A. et al. miR-802 regulates Paneth cell function and enterocyte differentiation in the mouse small intestine. Nat. Commun. 12, 3339 (2021).
Schell, J. C. et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 19, 1027–1036 (2017).
Mattila, J., Kokki, K., Hietakangas, V. & Boutros, M. Stem cell intrinsic hexosamine metabolism regulates intestinal adaptation to nutrient content. Dev. Cell 47, 112–121 (2018).
Crosnier, C. et al. Delta–Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132, 1093–1104 (2005).
Obniski, R., Sieber, M. & Spradling, A. C. Dietary lipids modulate Notch signaling and influence adult intestinal development and metabolism in Drosophila. Dev. Cell 47, 98–111 (2018).
Mana, M. D. et al. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 35, 109212 (2021).
Hinrichsen, F. et al. Microbial regulation of hexokinase 2 links mitochondrial metabolism and cell death in colitis. Cell Metab. 33, 2355–2366 (2021).
Frank, D. N. et al. Perilipin-2 modulates lipid absorption and microbiome responses in the mouse intestine. PLoS ONE 10, e0131944 (2015).
Lukonin, I. et al. Phenotypic landscape of intestinal organoid regeneration. Nature 586, 275–280 (2020).
Cheng, R., Ding, L., He, X., Takahashi, Y. & Ma, J. X. Interaction of PPARα with the canonic Wnt pathway in the regulation of renal fibrosis. Diabetes 65, 3730–3743 (2016).
de Araujo, I. E., Schatzker, M. & Small, D. M. Rethinking food reward. Annu. Rev. Psychol. 71, 139–164 (2020).
Tan, H. E. et al. The gut–brain axis mediates sugar preference. Nature 580, 511–516 (2020).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Liu, W. W. & Bohorquez, D. V. The neural basis of sugar preference. Nat. Rev. Neurosci. 23, 584–595 (2022).
Zhang, T., Perkins, M. H., Chang, H., Han, W. & de Araujo, I. E. An interorgan neural circuit for appetite suppression. Cell 185, 2478–2494 (2022).
Mithieux, G. The new functions of the gut in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 8, 445–449 (2005).
Soty, M., Gautier-Stein, A., Rajas, F. & Mithieux, G. Gut–brain glucose signaling in energy homeostasis. Cell Metab. 25, 1231–1242 (2017).
Vily-Petit, J. et al. Intestinal gluconeogenesis prevents obesity-linked liver steatosis and non-alcoholic fatty liver disease. Gut 69, 2193–2202 (2020).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014).
Park, S. Y. et al. Strain-level fitness in the gut microbiome is an emergent property of glycans and a single metabolite. Cell 185, 513–529 (2022).
Azais-Braesco, V., Sluik, D., Maillot, M., Kok, F. & Moreno, L. A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 16, 6 (2017).
Jang, C. et al. The small intestine shields the liver from fructose-induced steatosis. Nat. Metab. 2, 586–593 (2020).
Zhang, Z. et al. Adipocyte iron levels impinge on a fat-gut cross-talk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metab. 33, 1624–1639 (2021).
Rodriguez-Colman, M. J. et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–442 (2017).
Lee, Y. S. et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24, 833–846 (2018).
Hudry, B. et al. Sex differences in intestinal carbohydrate metabolism promote food intake and sperm maturation. Cell 178, 901–918 (2019).
Austin, K., Markovic, M. A. & Brubaker, P. L. Current and potential therapeutic targets of glucagon-like peptide 2. Curr. Opin. Pharmacol. 31, 13–18 (2016).
Speakman, J. R. & O’Rahilly, S. Fat: an evolving issue. Dis. Model Mech. 5, 569–573 (2012).
O’Rahilly, S. Human genetics illuminates the paths to metabolic disease. Nature 462, 307–314 (2009).
Purandare, A., Phalgune, D. & Shah, S. Variability of length of small intestine in Indian population and its correlation with type 2 diabetes mellitus and obesity. Obes. Surg. 29, 3149–3153 (2019).
Rohm, T. V. et al. Obesity in humans is characterized by gut inflammation as shown by pro-inflammatory intestinal macrophage accumulation. Front. Immunol. 12, 668654 (2021).
Monteiro-Sepulveda, M. et al. Jejunal T cell Inflammation in human obesity correlates with decreased enterocyte insulin signaling. Cell Metab. 22, 113–124 (2015).
Khan, S., Luck, H., Winer, S. & Winer, D. A. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat. Commun. 12, 2598 (2021).
Sullivan, Z. A. et al. γδ T cells regulate the intestinal response to nutrient sensing. Science https://doi.org/10.1126/science.aba8310 (2021).
Sukhotnik, I. et al. Dietary palmitic acid modulates intestinal re-growth after massive small bowel resection in a rat. Pediatr. Surg. Int. 24, 1313–1321 (2008).
Booth, I. W. Enteral nutrition as primary therapy in short-bowel syndrome. Gut 35, S69–S72 (1994).
Vanderhoof, J. A., Euler, A. R., Park, J. H. Y. & Grandjean, C. J. Augmentation of mucosal adaptation following massive small-bowel resection by 16,16-dimethyl-prostaglandin-E2 in the rat. Digestion 36, 213–219 (1987).
Leonard, M. M. et al. RNA sequencing of intestinal mucosa reveals novel pathways functionally linked to celiac disease pathogenesis. PLoS ONE 14, e0215132 (2019).
Jeppesen, P. B. et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 54, 1224–1231 (2005).
Bremholm, L., Hornum, M., Henriksen, B. M., Larsen, S. & Holst, J. J. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand. J. Gastroenterol. 44, 314–319 (2009).
Bruens, L., Ellenbroek, S. I. J., van Rheenen, J. & Snippert, H. J. In vivo imaging reveals existence of crypt fission and fusion in adult mouse intestine. Gastroenterology 153, 674–677 (2017).
Wang, D., Odle, J. & Liu, Y. Metabolic regulation of intestinal stem cell homeostasis. Trends Cell Biol. 31, 325–327 (2021).
Kieser, S., Zdobnov, E. M. & Trajkovski, M. Comprehensive mouse microbiota genome catalog reveals major difference to its human counterpart. PLoS Comput. Biol. 18, e1009947 (2022).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Abrams, G. D., Bauer, H. & Sprinz, H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab. Invest. 12, 355–364 (1963).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Perry, R. J. et al. Acetate mediates a microbiome–brain–beta cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Trajkovski, M. & Wollheim, C. B. Physiology: microbial signals to the brain control weight. Nature 534, 185–187 (2016).
McIntyre, N., Holdsworth, C. D. & Turner, D. S. New interpretation of oral glucose tolerance. Lancet 2, 20–21 (1964).
Kreuch, D. et al. Gut mechanisms linking intestinal sweet sensing to glycemic control. Front. Endocrinol. 9, 741 (2018).
Miguel-Aliaga, I. Nerveless and gutsy: intestinal nutrient sensing from invertebrates to humans. Semin. Cell Dev. Biol. 23, 614–620 (2012).
Margolskee, R. F. et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl Acad. Sci. USA 104, 15075–15080 (2007).
Sclafani, A., Koepsell, H. & Ackroff, K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R631–R639 (2016).
Groneberg, D. A., Doring, F., Eynott, P. R., Fischer, A. & Daniel, H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G697–G704 (2001).
Dranse, H. J. et al. Physiological and therapeutic regulation of glucose homeostasis by upper small intestinal PepT1-mediated protein sensing. Nat. Commun. 9, 1118 (2018).
Diakogiannaki, E. et al. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 56, 2688–2696 (2013).
Sivaprakasam, S. et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 5, e238 (2016).
Hirasawa, A. et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 (2005).
Zhao, L. et al. CD36 senses dietary lipids and regulates lipids homeostasis in the intestine. Front. Physiol. 12, 669279 (2021).
Li, M. et al. Gut–brain circuits for fat preference. Nature 610, 722–730 (2022).
Artegiani, B. et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR–Cas9 precision genome editing. Nat. Cell Biol. 22, 321–331 (2020).
Breau, K. A. et al. Efficient transgenesis and homology-directed gene targeting in monolayers of primary human small intestinal and colonic epithelial stem cells. Stem Cell Rep. 17, 1493–1506 (2022).
Zietek, T. et al. Organoids to study intestinal nutrient transport, drug uptake and metabolism—update to the human model and expansion of applications. Front. Bioeng. Biotechnol. 8, 577656 (2020).
Zietek, T., Rath, E., Haller, D. & Daniel, H. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci. Rep. 5, 16831 (2015).
Puschhof, J. et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 16, 4633–4649 (2021).
Co, J. Y. et al. Controlling epithelial polarity: a human enteroid model for host–pathogen interactions. Cell Rep. 26, 2509–2520 (2019).
Co, J. Y., Margalef-Catala, M., Monack, D. M. & Amieva, M. R. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat. Protoc. 16, 5171–5192 (2021).
Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020).
Goldspink, D. A. et al. Labeling and characterization of human GLP-1-secreting L cells in primary ileal organoid culture. Cell Rep. 31, 107833 (2020).
Aguilar, C. et al. Organoids as host models for infection biology—a review of methods. Exp. Mol. Med 53, 1471–1482 (2021).
Min, S., Kim, S. & Cho, S. W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med. 52, 227–237 (2020).
Acknowledgements
This work received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme, ERC advanced grant (787470, IntraGutSex), Biotechnology and Biological Sciences Research Council (BB000528/1), and MRC intramural funding to I.M.-A.; and from an ERC consolidator grant (815962, Healthybiota), the Swiss National Science Foundation (grant 310030_205042) and the Clayton Foundation for Biomedical Research to M.T.
Author information
Authors and Affiliations
Contributions
All authors conceptualized, discussed and wrote the paper. O.S. prepared the figures. M.T. initiated the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Anika Boettcher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stojanović, O., Miguel-Aliaga, I. & Trajkovski, M. Intestinal plasticity and metabolism as regulators of organismal energy homeostasis. Nat Metab 4, 1444–1458 (2022). https://doi.org/10.1038/s42255-022-00679-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00679-6