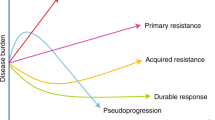
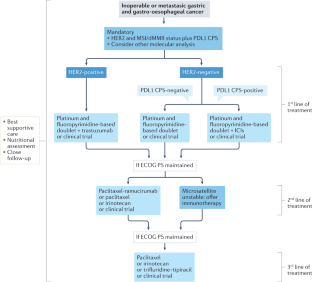
Abstract
Gastric cancer and gastro-oesophageal junction cancer represent a global health-care challenge. Despite the efficacy of improved chemotherapy and surgical options, these patients still have a poor prognosis. In advanced disease, only trastuzumab and some immune checkpoint inhibitors, such as nivolumab and pembrolizumab in addition to chemotherapy, have demonstrated consistent and reliable efficacy in patients with HER2-positive and PDL1-positive tumours, respectively. In this Review, we discuss the intrinsic characteristics of gastric and gastro-oesophageal cancer from the molecular and clinical perspectives and provide a comprehensive review of previously reported and ongoing phase II and III clinical trials with targeted agents and immunotherapy in advanced and localized settings. Finally, we suggest alternative strategies to help overcome current challenges in precision medicine and to improve outcomes for these patients.
Key points
-
The spatial and temporal heterogeneity features of gastric and gastro-oesophageal tumours have jeopardized the success of most phase II and III clinical trials with targeted treatments.
-
Current biomarkers for treatment decisions in patients with gastric and gastro-oesophageal cancer include testing for HER2 overexpression and amplification, PDL1 combined positive score expression and microsatellite instability-high status.
-
Chemotherapy drugs of reduced toxicity, together with some molecularly driven targeted therapies, have been shown to be particularly valuable for sequential treatment strategies in optimizing the survival of patients with gastric and gastro-oesophageal cancer.
-
National strategies with massive molecular screening of patients with gastric and gastro-oesophageal cancer have emerged as promising approaches to detect those candidates who could benefit from targeted treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21660 (2021).
Bosman, F. T., Carneiro, F., Hruban, R. H. & Theise, N. D. WHO classification of tumours of the digestive system: WHO Classification of Tumours 4th edn. Vol. 3 (IARC, 2010).
Bass, A. J. et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Cristescu, R. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 21, 449–456 (2015).
Thun, M. J., Wild, C. P. & Colditz, G. in Schottenfeld Fraumeni Cancer Epidemiology and Prevention 4th edn (eds Thun, M. J., Linet, M. S., Cerhan, J. R., Haiman, C. A. & Schottenfeld, D.) 1193–1204 (Oxford Univ. Press, 2017).
The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017).
Lordick, F. et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. https://doi.org/10.1016/J.ANNONC.2022.07.004 (2022).
American Cancer Society. Cancer facts & figures 2021 (American Cancer Society, 2021).
Davidson, M. et al. Survival in advanced esophagogastric adenocarcinoma improves with use of multiple lines of therapy: results from an analysis of more than 500 patients. Clin Colorectal Cancer 17, 223–230 (2018).
Fanotto, V. et al. Outcomes of advanced gastric cancer patients treated with at least three lines of systemic chemotherapy. Oncologist 22, 1463–1469 (2017).
Cafferkey, C. et al. Survival in advanced oesophagogastric adenocarcinoma (OGA) improves with the use of multiple lines of therapy: results from an analysis of over 500 patients (pts) [abstract 642P]. Ann. Oncol. 28 (Suppl. 5), v219 (2017).
Hess, L. M. et al. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer 19, 607–615 (2016).
Deng, N. et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61, 673–684 (2012).
Dulak, A. M. et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 72, 4383–4393 (2012).
Sohn, B. H. et al. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas project. Clin. Cancer Res. 23, 4441–4449 (2017).
Janjigian, Y. Y. et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 8, 49–58 (2018).
Suh, Y. S. et al. Comprehensive molecular characterization of adenocarcinoma of the gastroesophageal junction between esophageal and gastric adenocarcinomas. Ann. Surg. 275, 706–717 (2022).
Tabernero, J. et al. End-of-study analysis from JACOB: A phase III study of pertuzumab (P) + trastuzumab (H) and chemotherapy (CT) in HER2-positive metastatic gastric or gastro-esophageal junction cancer (mGC/GEJC) [abstract 1423MO]. Ann. Oncol. 31 (Suppl. 4), S900–S901 (2020).
Satoh, T. et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J. Clin. Oncol. 32, 2039–2049 (2014).
Hecht, J. R. et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J. Clin. Oncol. 34, 443–451 (2016).
Thuss-Patience, P. C. et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 18, 640–653 (2017).
Waddell, T. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 481–489 (2013).
Lordick, F. et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 490–499 (2013).
Dutton, S. J. et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 15, 894–904 (2014).
Catenacci, D. V. T. et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1467–1482 (2017).
Shah, M. A. et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric Randomized Clinical Trial. JAMA Oncol. 3, 620–627 (2017).
Alsina, M., Gullo, I. & Carneiro, F. Intratumoral heterogeneity in gastric cancer: a new challenge to face. Ann. Oncol. 28, 912–913 (2017).
Nakamura, Y., Kawazoe, A., Lordick, F., Janjigian, Y. Y. & Shitara, K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat. Rev. Clin. Oncol. 18, 473–487 (2021).
Pectasides, E. et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 8, 37–48 (2018).
Gullo, I., Carneiro, F., Oliveira, C. & Almeida, G. M. Heterogeneity in gastric cancer: from pure morphology to molecular classifications. Pathobiology 85, 50–63 (2018).
Catenacci, D. V. T. et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): a phase II study evaluating an individualized treatment strategy for metastatic disease. Cancer Discov. 11, 308–325 (2021).
Nakamura, Y. et al. Emergence of concurrent multiple EGFR mutations and MET amplification in a patient with EGFR-amplified advanced gastric cancer treated with cetuximab. JCO Precis. Oncol. 4, 1407–1413 (2020).
Parikh, A. R. et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 25, 1415–1421 (2019).
Marshall, B. J. & Warren, J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 323, 1311–1315 (1984).
Engstrand, L. & Graham, D. Y. Microbiome and gastric cancer. Dig. Dis. Sci. 65, 865–873 (2020).
Yang, J., Zhou, X., Liu, X., Ling, Z. & Ji, F. Role of the gastric microbiome in gastric cancer: from carcinogenesis to treatment. Front. Microbiol. 12, 641322 (2021).
Wu, F. et al. Oral and gastric microbiome in relation to gastric intestinal metaplasia. Int. J. Cancer 150, 928–940 (2022).
Ferreira, R. M. et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67, 226–236 (2018).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393, 1948–1957 (2019).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20 (2006).
Smyth, E. C. et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (Suppl. 5), v38–v49 (2016).
Thuss-Patience, P. C. et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 47, 2306–2314 (2011).
Ford, H. E. R. et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 15, 78–86 (2014).
Iizumi, S., Takashima, A., Sakamaki, K., Morita, S. & Boku, N. Survival impact of post-progression chemotherapy in advanced gastric cancer: systematic review and meta-analysis. Cancer Chemother. Pharmacol. 81, 981–989 (2018).
Cunningham, D. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358, 36–46 (2008).
Al-Batran, S. E. et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 26, 1435–1442 (2008).
Hall, P. S. et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 7, 869–877 (2021).
Bang, Y.-J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Sun, J. M. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398, 759–771 (2021).
Tabernero, J. et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 19, 1372–1384 (2018).
Ohtsu, A. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29, 3968–3976 (2011).
Fuchs, C. S. et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 420–435 (2019).
Rivera, F. et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur. J. Cancer 145, 158–167 (2021).
Al-Batran, S.-E. et al. Final results and subgroup analysis of the PETRARCA randomized phase II AIO trial: Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma [abstract 1421MO]. Ann. Oncol. 31 (Suppl. 4), S899 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02205047 (2022).
Bartley, A. N. et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35, 446–464 (2017).
Haffner, I. et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J. Clin. Oncol. 39, 1468–1478 (2021).
Gomez-Martin, C. et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J. Clin. Oncol. 31, 4445–4452 (2013).
Kaito, A. et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J. Clin. Cases 7, 1964–1977 (2019).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Invest. 124, 5145–5158 (2014).
Augustin, J. E., Soussan, P. & Bass, A. J. Targeting the complexity of ERBB2 biology in gastroesophageal carcinoma. Ann. Oncol. https://doi.org/10.1016/J.ANNONC.2022.08.001 (2022).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04704934 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03602079 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03821233 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03255070 (2022).
Bang, Y. J. et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 28, 855–861 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04499924 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05152147 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03615326 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04379596 (2022).
Catenacci, D. V. T. et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 21, 1066–1076 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04082364 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04430738 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04276493 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03650348 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05190445?term=NCT05190445&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04639219 (2022).
Yamaguchi, K. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-low, advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: results of the exploratory cohorts in the phase II, multicenter, open-label DESTINY-Gastric01 study [abstract 1422MO]. Ann. Oncol. 31 (Suppl. 4), S899–S900 (2020).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Kang, Y.-K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471 (2017).
Fuchs, C. S. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4, e180013 (2018).
Bang, Y. J. et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann. Oncol. 29, 2052–2060 (2018).
Shitara, K. et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04508140 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04879368?term=NCT04879368&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04752358 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04594811 (2022).
Shitara, K. et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 6, 1571–1580 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03675737 (2022).
Kang, Y. K. et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23, 234–247 (2022).
Xu, J. et al. Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study [abstract LBA53]. Ann. Oncol. 32 (Suppl. 5), S1331 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03777657 (2022).
Xu, R. et al. RATIONALE 305: tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line therapy in patients with gastric or gastroesophageal junction adenocarcinoma [abstract P-26]. Ann. Oncol. 31 (Suppl. 3), S97–S98 (2020).
Moehler, M. et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results From JAVELIN Gastric 100. J. Clin. Oncol. 39, 966–977 (2021).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10 (2020).
Chao, J. et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 7, 895–902 (2021).
Janjigian, Y. et al. Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study [abstract LBA7]. Ann. Oncol. 32 (Suppl. 5), S1329–S1330 (2021).
Kim, S. T. et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 24, 1449–1458 (2018).
Shitara, K. et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061 [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4537 (2020).
Khalid, S. et al. Association of TMB using the Foundation Medicine Companion Diagnostic (F1CDx) with efficacy of first-line pembrolizumab (pembro) or pembro plus chemotherapy (pembro + chemo) versus chemo in patients with gastric cancer (gc) from KEYNOTE-062 [abstract 1442P]. Ann. Oncol. 31 (Suppl. 4), S907–S908 (2020).
Janjigian, Y. et al. Co-occurring HER2 and PD-L1 expression in patients with HER2-positive trastuzumab-refractory gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): biomarker analysis from the trastuzumab deruxtecan (T-DXd) DESTINY-Gastric03 trial [abstract SO-7]. Ann. Oncol. 33 (Suppl. 4), S358–S359 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04662710 (2022).
Palmer, A. C., Izar, B., Hwangbo, H. & Sorger, P. K. Predictable clinical benefits without evidence of synergy in trials of combination therapies with immune-checkpoint inhibitors. Clin. Cancer Res. 28, 368–377 (2022).
Ji, J. et al. AK104 (PD-1/CTLA-4 bispecific) combined with chemotherapy as first-line therapy for advanced gastric (G) or gastroesophageal junction (GEJ) cancer: updated results from a phase Ib study [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 232 (2021).
Kamada, T. et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116, 9999–10008 (2019).
Liu, Y. et al. Camrelizumab combined with FOLFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4536 (2020).
Li, H. et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 4050 (2021).
Alcindor, T. et al. Phase II trial of perioperative chemotherapy + avelumab in locally advanced gastroesophageal adenocarcinoma: Preliminary results [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 4046 (2021).
Alsina, M. et al. MONEO: a phase II study of avelumab (Av) plus FLOT in the peri-operative treatment for patients (pts) with resectable gastric or gastroesophageal junction cancer (GC) [abstract]. J. Clin. Oncol. 39 (Suppl. 15), TPS4155 (2021).
Bang, Y. J. et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Futur. Oncol. 15, 943–952 (2019).
Janjigian, Y. Y. et al. MATTERHORN: efficacy and safety of neoadjuvant-adjuvant durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer–a randomized, double-blind, placebo-controlled, phase 3 study [abstract]. J. Clin. Oncol. 39 (Suppl. 15), TPS4151 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04745988?term=NCT04745988&draw=2&rank=1 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03443856?term=NCT03443856&draw=2&rank=1 (2022).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 384, 1191–1203 (2021).
André, T. et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J. Clin. Oncol. https://doi.org/10.1200/JCO.22.00686 (2022).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Fuchs, C. S. et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383, 31–39 (2014).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–1235 (2014).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454 (2016).
Kang, Y.-K. et al. Randomized phase III ANGEL study of rivoceranib (apatinib)+best supportive care (BSC) vs placebo+BSC in patients with advanced/metastatic gastric cancer who failed ≥2 prior chemotherapy regimens [abstract LBA43]. Ann. Oncol. 30 (Suppl. 5), v877–v878 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05029453?term=NCT05029453&draw=2&rank=1 (2021).
Al-Batran, S.-E. et al. Perioperative FLOT plus ramucirumab versus FLOT alone for resectable esophagogastric adenocarcinoma–updated results and subgroup analyses of the randomized phase II/III trial RAMSES/FLOT7 of the German AIO and Italian GOIM [abstract 1424MO]. Ann. Oncol. 31 (Suppl. 4), S901 (2020).
Van Cutsem, E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 30, 2119–2127 (2012).
Maron, S. B. et al. Targeted therapies for targeted populations: anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 8, 696–713 (2018).
Sahasrabudhe, R. et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology 152, 983–986.e6 (2017).
Bang, Y. J. et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1637–1651 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03427814 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02678182 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04209686?term=NCT04209686&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03008278 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03995017 (2022).
Lang, S. A. et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int. J. Cancer 120, 1803–1810 (2007).
Lee, J. et al. Phase II trial of capecitabine and everolimus (RAD001) combination in refractory gastric cancer patients. Invest. New Drugs 31, 1580–1586 (2013).
Ohtsu, A. et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J. Clin. Oncol. 31, 3935–3943 (2013).
Kim, H. S., Kim, J. H. & Jang, H. J. Pathologic and prognostic impacts of FGFR2 amplification in gastric cancer: a meta-analysis and systemic review. J. Cancer 10, 2560–2567 (2019).
Catenacci, D. V. T. et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J. Clin. Oncol. 38, 2418–2426 (2020).
Catenacci, D. V. T. et al. FIGHT: A randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b + advanced gastric/gastroesophageal junction adenocarcinoma (GC) [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 4010 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05052801 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05111626 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04604132 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02052778 (2022).
Van Cutsem, E. et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 28, 1316–1324 (2017).
Hashimoto, I. & Oshima, T. Claudins and gastric cancer: an overview. Cancers 14, 290 (2022).
Sahin, U. et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 32, 609–619 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03653507 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03504397 (2022).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03874897 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04400383?term=NCT04400383&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04404595 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04467853?term=NCT04467853&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04260191 (2022).
Alsina, M., Diez, M. & Tabernero, J. Emerging biological drugs for the treatment of gastroesophageal adenocarcinoma. Expert. Opin. Emerg. Drugs 26, 385–400 (2021).
Lee, J. et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the Viktory Umbrella Trial. Cancer Discov. 9, 1388–1405 (2019).
Yuki, S. et al. The nationwide cancer genome screening project in Japan SCRUM-Japan GI-SCREEN: efficient identification of cancer genome alterations in advanced gastric cancer (GC) [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 4050 (2018).
Nakamura, Y. et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 26, 1859–1864 (2020).
Wainberg, Z. A. et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT) [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 160 (2021).
Dykewicz, C. A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 33, 139–144 (2001).
Author information
Authors and Affiliations
Contributions
M.A., V.A. and M.D. researched data for the article. M.A. and V.A. contributed substantially to discussion of the content. M.A., V.A. and M.D. wrote the article. M.A., V.A. and J.T. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.A. declares consulting/advisory roles with BMS, Lilly, MSD and Servier; and honoraria for speaking from Amgen, BMS, Lilly, MSD and Servier. V.A. declares consulting/advisory roles with BMS and MSD; and honoraria for speaking from BMS and Lilly. M.D. reports a financial interest in the form of a scientific consultancy role for Lilly and travel expenses partially covered by Lilly. J.T. reports a personal financial interest in the form of a scientific consultancy role for Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho, Tessa Therapeutics and TheraMyc; and also educational collaborations with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER); and declares institutional financial interests in the form of financial support for clinical trials or contracted research for Amgen Inc, Array Biopharma Inc, AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, BMS, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc, Spanish Association Against Cancer Scientific Foundation and Cancer Research UK.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Andres Cervantes, Yukinori Kurokawa and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsina, M., Arrazubi, V., Diez, M. et al. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol 20, 155–170 (2023). https://doi.org/10.1038/s41575-022-00703-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00703-w
This article is cited by
-
MBD3 promotes epithelial-mesenchymal transition in gastric cancer cells by upregulating ACTG1 via the PI3K/AKT pathway
Biological Procedures Online (2024)
-
LncRNA WFDC21P interacts with SEC63 to promote gastric cancer malignant behaviors by regulating calcium homeostasis signaling pathway
Cancer Cell International (2024)
-
GGT5 facilitates migration and invasion through the induction of epithelial–mesenchymal transformation in gastric cancer
BMC Medical Genomics (2024)
-
Construction of a gene model related to the prognosis of patients with gastric cancer receiving immunotherapy and exploration of COX7A1 gene function
European Journal of Medical Research (2024)
-
Machine learning to establish three sphingolipid metabolism genes signature to characterize the immune landscape and prognosis of patients with gastric cancer
BMC Genomics (2024)