Abstract
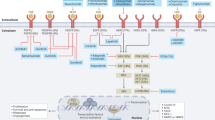
Advances in cancer biology and sequencing technology have enabled the selection of targeted and more effective treatments for individual patients with various types of solid tumour. However, only three molecular biomarkers have thus far been demonstrated to predict a response to targeted therapies in patients with gastric and/or gastro-oesophageal junction (G/GEJ) cancers: HER2 positivity for trastuzumab and trastuzumab deruxtecan, and microsatellite instability (MSI) status and PD-L1 expression for pembrolizumab. Despite this lack of clinically relevant biomarkers, distinct molecular subtypes of G/GEJ cancers have been identified and have informed the development of novel agents, including receptor tyrosine kinase inhibitors and monoclonal antibodies, several of which are currently being tested in ongoing trials. Many of these trials include biomarker stratification, and some include analysis of circulating tumour DNA (ctDNA), which both enables the noninvasive assessment of biomarker expression and provides an indication of the contributions of intratumoural heterogeneity to response and resistance. The results of these studies might help to optimize the selection of patients to receive targeted therapies, thus facilitating precision medicine approaches for patients with G/GEJ cancers. In this Review, we describe the current evidence supporting the use of targeted therapies in patients with G/GEJ cancers and provide guidance on future research directions.
Key points
-
Molecular subtypes, such as those provided by The Cancer Genome Atlas (TCGA), based on molecular profiling of gastric and gastro-oesophageal junction (G/GEJ) cancers are associated with distinct molecular and clinical characteristics.
-
Molecular heterogeneity is a major reason for the frequent failure of biomarker-based clinical trials in patients with G/GEJ cancers and can be assessed by analysis of circulating tumour DNA (ctDNA).
-
Novel agents, including receptor tyrosine kinase inhibitors, monoclonal antibodies and antibody–drug conjugates, are currently being tested in ongoing biomarker-guided trials.
-
Sequencing of both tumour tissue DNA and ctDNA can be used for the identification of targetable alterations, including rare alterations, thus enabling precision medicine approaches for patients with G/GEJ cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Glimelius, B. et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann. Oncol. 8, 163–168 (1997).
Thuss-Patience, P. C. et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the arbeitsgemeinschaft internistische onkologie (AIO). Eur. J. Cancer 47, 2306–2314 (2011).
Kang, J. H. et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 30, 1513–1518 (2012).
Ford, H. E. et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 15, 78–86 (2014).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Fuchs, C. S. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4, e180013 (2018).
Kang, Y.-K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471 (2017).
Moehler, M. et al. LBA6_PR. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Ann. Oncol. 31, S1191 (2020).
El-Deiry, W. S. et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J. Clin. 69, 305–343 (2019).
Lauren, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 64, 31–49 (1965).
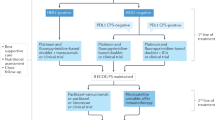
Lee, J. et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY umbrella trial. Cancer Discov. 9, 1388–1405 (2019).
Nakamura, Y. & Shitara, K. Development of circulating tumour DNA analysis for gastrointestinal cancers. ESMO Open 5, e000600 (2020).
Hutter, C. & Zenklusen, J. C. The Cancer Genome Atlas: creating lasting value beyond its data. Cell 173, 283–285 (2018).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Yanai, H. et al. Endoscopic and pathologic features of Epstein-Barr virus-associated gastric carcinoma. Gastrointest. Endosc. 45, 236–242 (1997).
van Beek, J. et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J. Clin. Oncol. 22, 664–670 (2004).
Camargo, M. C. et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut 63, 236–243 (2014).
Koh, J. et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget 8, 26356–26367 (2017).
Kawazoe, A. et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 20, 407–415 (2017).
Kang, G. H. et al. Epstein-Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am. J. Pathol. 160, 787–794 (2002).
Kaneda, A., Matsusaka, K., Aburatani, H. & Fukayama, M. Epstein-Barr virus infection as an epigenetic driver of tumorigenesis. Cancer Res. 72, 3445–3450 (2012).
Oki, E., Oda, S., Maehara, Y. & Sugimachi, K. Mutated gene-specific phenotypes of dinucleotide repeat instability in human colorectal carcinoma cell lines deficient in DNA mismatch repair. Oncogene 18, 2143–2147 (1999).
Vilar, E. & Gruber, S. B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 7, 153–162 (2010).
Latham, A. et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J. Clin. Oncol. 37, 286–295 (2019).
Gylling, A. et al. Is gastric cancer part of the tumour spectrum of hereditary non-polyposis colorectal cancer? A molecular genetic study. Gut 56, 926 (2007).
Ma, C. et al. Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein-Barr virus or microsatellite instability. Am. J. Surg. Pathol. 40, 1496–1506 (2016).
Liu, Y. et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 33, 721–735 e728 (2018).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Thumkeo, D., Watanabe, S. & Narumiya, S. Physiological roles of Rho and Rho effectors in mammals. Eur. J. Cell Biol. 92, 303–315 (2013).
Yao, F. et al. Recurrent fusion genes in gastric cancer: CLDN18-ARHGAP26 induces loss of epithelial integrity. Cell Rep. 12, 272–285 (2015).
Cho, S. Y. et al. Sporadic early-onset diffuse gastric cancers have high frequency of somatic CDH1 alterations, but low frequency of somatic RHOA mutations compared with late-onset cancers. Gastroenterology 153, 536–549 e526 (2017).
Bajrami, I. et al. E-cadherin/ROS1 inhibitor synthetic lethality in breast cancer. Cancer Discov. 8, 498–515 (2018).
Zhang, H. et al. Gain-of-function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 10, 288–305 (2020).
Sohn, B. H. et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-16-2211 (2017).
Janjigian, Y. Y. et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 8, 49–58 (2018).
Kubota, Y. et al. The impact of molecular subtype on efficacy of chemotherapy and checkpoint inhibition in advanced gastric cancer. Clin. Cancer Res. 26, 3784–3790 (2020).
Cristescu, R. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 21, 449–456 (2015).
Kim, S. T. et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 24, 1449–1458 (2018).
Sathe, A. et al. Single-cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin. Cancer Res. 26, 2640–2653 (2020).
Mun, D. G. et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell 35, 111–124 e110 (2019).
Hecht, J. R. et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J. Clin. Oncol. 34, 443–451 (2016).
Tabernero, J. et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 19, 1372–1384 (2018).
Satoh, T. et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J. Clin. Oncol. 32, 2039–2049 (2014).
Thuss-Patience, P. C. et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 18, 640–653 (2017).
Van Cutsem, E. et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 28, 1316–1324 (2017).
Waddell, T. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 481–489 (2013).
Lordick, F. et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 490–499 (2013).
Dutton, S. J. et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 15, 894–904 (2014).
Catenacci, D. V. T. et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1467–1482 (2017).
Shah, M. A. et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 3, 620–627 (2017).
Hofmann, M. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52, 797–805 (2008).
Kurokawa, Y. et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer 18, 691–697 (2015).
Lee, S., de Boer, W. B., Fermoyle, S., Platten, M. & Kumarasinghe, M. P. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology 59, 832–840 (2011).
Lee, H. E. et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur. J. Cancer 49, 1448–1457 (2013).
Wang, T. et al. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum. Pathol. 45, 970–975 (2014).
Ahn, S. et al. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget 6, 38372–38380 (2015).
Van Cutsem, E. et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18, 476–484 (2015).
Yagi, S. et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer 22, 518–525 (2019).
Kaito, A. et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J. Clin. Cases 7, 1964–1977 (2019).
Albarello, L., Pecciarini, L. & Doglioni, C. HER2 testing in gastric cancer. Adv. Anat. Pathol. 18, 53–59 (2011).
Pirrelli, M., Caruso, M. L., Di Maggio, M., Armentano, R. & Valentini, A. M. Are biopsy specimens predictive of HER2 status in gastric cancer patients? Dig. Dis. Sci. 58, 397–404 (2013).
Wakatsuki, T. et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J. Gastroenterol. 53, 1186–1195 (2018).
Marx, A. H. et al. HER-2 amplification is highly homogenous in gastric cancer. Hum. Pathol. 40, 769–777 (2009).
Kim, M. A., Lee, H. J., Yang, H. K., Bang, Y. J. & Kim, W. H. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 59, 822–831 (2011).
Bozzetti, C. et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br. J. Cancer 104, 1372–1376 (2011).
Fassan, M. et al. Human epithelial growth factor receptor 2 (HER2) status in primary and metastatic esophagogastric junction adenocarcinomas. Hum. Pathol. 43, 1206–1212 (2012).
Fusco, N. et al. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod. Pathol. 26, 816–824 (2013).
Cho, E. Y. et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod. Pathol. 26, 677–684 (2013).
Cappellesso, R. et al. HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum. Pathol. 46, 665–672 (2015).
Park, S. R. et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur. J. Cancer 53, 42–50 (2016).
Pietrantonio, F. et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. Int. J. Cancer 139, 2859–2864 (2016).
Seo, S. et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer 22, 527–535 (2019).
Kurokawa, Y. et al. Prognostic impact of major receptor tyrosine kinase expression in gastric cancer. Ann. Surg. Oncol. 21 (Suppl. 4), S584–S590 (2014).
Nagatsuma, A. K. et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 18, 227–238 (2015).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Invest. 124, 5145–5158 (2014).
Lee, J. Y. et al. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci. Rep. 5, 9289 (2015).
Nakamura, Y. & Yoshino, T. Clinical utility of analyzing circulating tumor DNA in patients with metastatic colorectal cancer. Oncologist 23, 1310–1318 (2018).
Wang, D. S. et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 68, 1152–1161 (2019).
Sanchez-Vega, F. et al. EGFR and MET amplifications determine response to HER2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 9, 199–209 (2019).
Pectasides, E. et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 8, 37–48 (2018).
Maron, S. B. et al. Targeted therapies for targeted populations: anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 8, 696–713 (2018).
Nakamura, Y. et al. Emergence of concurrent multiple EGFR mutations and MET amplification in a patient with EGFR-amplified advanced gastric cancer treated with cetuximab. JCO Precis. Oncol. 4, PO.20.00263 (2020).
Parikh, A. R. et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 25, 1415–1421 (2019).
Kwak, E. L. et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-amplified esophagogastric cancer. Cancer Discov. 5, 1271–1281 (2015).
Wainberg, Z. A. et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). J Clin Oncol 39:3_suppl, 160-160 (2021).
Strickler, J. H. et al. MOUNTAINEER-02: Phase II/III study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma — Trial in Progress. J Clin Oncol 39:3_suppl, TPS252-TPS252 (2021).
Derks, S. et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann. Oncol. 31, 1011–1020 (2020).
Lin, S. J. et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 64, 1721–1731 (2015).
Yano, T. et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol. Rep. 15, 65–71 (2006).
Park, D. I. et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig. Dis. Sci. 51, 1371–1379 (2006).
Giuffre, G., Ieni, A., Barresi, V., Caruso, R. A. & Tuccari, G. HER2 status in unusual histological variants of gastric adenocarcinomas. J. Clin. Pathol. 65, 237–241 (2012).
Muro, K. et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 30, 19–33 (2019).
NCCN. NCCN guidelines Version 2. 2021 Gastric Cancer https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (2021).
Shah, M. A. et al. Biomarker analysis of the GATSBY study of trastuzumab emtansine versus a taxane in previously treated HER2-positive advanced gastric/gastroesophageal junction cancer. Gastric Cancer 22, 803–816 (2019).
Kim, S. T. et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann. Oncol. 29, 1037–1048 (2018).
Doi, T. et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 18, 1512–1522 (2017).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Yamaguchi, K. et al. 1422MO. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-low, advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Results of the exploratory cohorts in the phase II, multicenter, open-label DESTINY-Gastric01 study. Ann. Oncol. 31, S899–S900 (2020).
Gall, V. A. et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. 77, 5374–5383 (2017).
Iwata, T. N., Sugihara, K., Wada, T. & Agatsuma, T. [Fam-]trastuzumab deruxtecan (DS-8201a)-induced antitumor immunity is facilitated by the anti-CTLA-4 antibody in a mouse model. PLoS ONE 14, e0222280 (2019).
Varadan, V. et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperativetrastuzumab and chemotherapy in HER2-positive early breast cancer. Clin. Cancer Res. 22, 3249–3259 (2016).
Chaganty, B. K. R. et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 430, 47–56 (2018).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011).
Janjigian, Y. Y. et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 21, 821–831 (2020).
Janjigian, Y. Y. et al. KEYNOTE-811 pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction cancer (mG/GEJC): a double-blind, randomized, placebo-controlled phase 3 study. J. Clin. Oncol. 37, TPS4146–TPS4146 (2019).
Catenacci, D. V. T. et al. Antitumor activity of margetuximab (M) plus pembrolizumab (P) in patients (pts) with advanced HER2+ (IHC3+) gastric carcinoma (GC). J. Clin. Oncol. 37, 65–65 (2019).
Catenacci, D. V. T. et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 21, 1066–1076 (2020).
Kulukian, A. et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 19, 976–987 (2020).
No authors listed. ZW25 effective in HER2-positive cancers. Cancer Discov. 9, 8 (2019).
Weisser, N., Wickman, G., Davies, R. & Rowse, G. Abstract 31. Preclinical development of a novel biparatopic HER2 antibody with activity in low to high HER2 expressing cancers. Cancer Res. 77, 31 (2017).
Meric-Bernstam F, et al. Zanidatamab (ZW25) in HER2-expressing gastroesophageal adenocarcinoma (GEA): Results from a phase I study. DOI: 10.1200/JCO.2021.39.3_suppl.164 Journal of Clinical Oncology 39, no. 3_suppl (January 20, 2021) 164-164.
Jung, E. J., Jung, E. J., Min, S. Y., Kim, M. A. & Kim, W. H. Fibroblast growth factor receptor 2 gene amplification status and its clinicopathologic significance in gastric carcinoma. Hum. Pathol. 43, 1559–1566 (2012).
Helsten, T. et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 22, 259–267 (2016).
Kuboki, Y. et al. In situ analysis of FGFR2 mRNA and comparison with FGFR2 gene copy number by dual-color in situ hybridization in a large cohort of gastric cancer patients. Gastric Cancer 21, 401–412 (2018).
Pearson, A. et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 6, 838–851 (2016).
Jang, J. et al. Antitumor effect of AZD4547 in a fibroblast growth factor receptor 2-amplified gastric cancer patient-derived cell model. Transl. Oncol. 10, 469–475 (2017).
Cha, Y. et al. FGFR2 amplification is predictive of sensitivity to regorafenib in gastric and colorectal cancers in vitro. Mol. Oncol. 12, 993–1003 (2018).
Goyal, L. et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 9, 1064–1079 (2019).
Bahleda, R. et al. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann. Oncol. 31, 1405–1412 (2020).
Hollebecque, A. et al. A phase II study of futibatinib (TAS-120) in patients (pts) with advanced (adv) solid tumors harboring fibroblast growth factor receptor (FGFR) genomic aberrations. J. Clin. Oncol. 38, TPS470–TPS470 (2020).
Catenacci, D. V. T. et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J. Clin. Oncol. 38, 2418–2426 (2020).
Catenacci, D. V. et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT phase III study design. Future Oncol. 15, 2073–2082 (2019).
Liao, J. B., Lee, H. P., Fu, H. T. & Lee, H. S. Assessment of EGFR and ERBB2 (HER2) in gastric and gastroesophageal carcinomas: EGFR amplification is associated with a worse prognosis in early stage and well to moderately differentiated carcinoma. Appl. Immunohistochem. Mol. Morphol. 26, 374–382 (2018).
Lordick, F. et al. Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study. J. Clin. Oncol. 31, 4021–4021 (2013).
Petty, R. D. et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J. Clin. Oncol. 35, 2279–2287 (2017).
Montagut, C. et al. Efficacy of Sym004 in patients with metastatic colorectal cancer with acquired resistance to anti-EGFR therapy and molecularly selected by circulating tumor DNA analyses: a phase 2 randomized clinical trial. JAMA Oncol. 4, e175245 (2018).
Kato, S. et al. Revisiting epidermal growth factor receptor (EGFR) amplification as a target for anti-EGFR therapy: analysis of cell-free circulating tumor DNA in patients with advanced malignancies. JCO Precis. Oncol. 3, PO.18.00180 (2019).
Schmees, N. et al. Abstract 4454. Identification of BAY-218, a potent and selective small-molecule AhR inhibitor, as a new modality to counteract tumor immunosuppression. Cancer Res. 79, 4454 (2019).
Van Cutsem, E. et al. A multicenter phase II study of AMG 337 in patients with MET-amplified gastric/gastroesophageal junction/esophageal adenocarcinoma and other MET-amplified solid tumors. Clin. Cancer Res. 25, 2414–2423 (2019).
Lee, J. et al. Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget 6, 28211–28222 (2015).
Guo, R. et al. MET-dependent solid tumours - molecular diagnosis and targeted therapy. Nat. Rev. Clin. Oncol. 17, 569–587 (2020).
Frigault, M. M. et al. Mechanisms of acquired resistance to savolitinib, a selective MET inhibitor in MET-amplified gastric cancer. JCO Precis. Oncol. 4, PO.19.00386 (2020).
Yuan, F. et al. Capecitabine metronomic chemotherapy inhibits the proliferation of gastric cancer cells through anti-angiogenesis. Oncol. Rep. 33, 1753–1762 (2015).
Zhang, Y. et al. Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy. Proc. Natl Acad. Sci. USA 114, E5226–E5235 (2017).
Ohtsu, A. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29, 3968–3976 (2011).
Shen, L. et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 18, 168–176 (2015).
Fuchs, C. S. et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383, 31–39 (2014).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–1235 (2014).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454 (2016).
Kang, Y. K. et al. Randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed ≥2 prior chemotherapy regimens. Ann. Oncol. 30, v877–v878 (2019).
Van Cutsem, E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 30, 2119–2127 (2012).
Van Cutsem, E. et al. Biomarker analyses of second-line ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. Eur. J. Cancer 127, 150–157 (2020).
Fuchs, C. S. et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br. J. Cancer 115, 974–982 (2016).
Alexandrov, L. B., Nik-Zainal, S., Siu, H. C., Leung, S. Y. & Stratton, M. R. A mutational signature in gastric cancer suggests therapeutic strategies. Nat. Commun. 6, 8683 (2015).
Sahasrabudhe, R. et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology 152, 983–986 e986 (2017).
Smyth, E. C. et al. Genomic loss of heterozygosity and survival in the REAL3 trial. Oncotarget 9, 36654–36665 (2018).
Bang, Y. J. et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1637–1651 (2017).
Liu, Y. Z. et al. Olaparib plus paclitaxel sensitivity in biomarker subgroups of gastric cancer. Ann. Oncol. 29, viii25–viii26 (2018).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377, 523–533 (2017).
Moore, K. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 379, 2495–2505 (2018).
Golan, T. et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 381, 317–327 (2019).
de Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Penson, R. T. et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J. Clin. Oncol. 38, 1164–1174 (2020).
Jiao, S. et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin. Cancer Res. 23, 3711–3720 (2017).
Sen, T. et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 9, 646–661 (2019).
Domchek, S. M. et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 21, 1155–1164 (2020).
Lampert, E. J. et al. Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: a proof-of-concept phase II study. Clin. Cancer Res. 26, 4268–4279 (2020).
Mathews, M. T. & Berk, B. C. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler. Thromb. Vasc. Biol. 28, 711–717 (2008).
Liu, J. F. et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 15, 1207–1214 (2014).
Niimi, T. et al. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell Biol. 21, 7380–7390 (2001).
Sahin, U. et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 14, 7624–7634 (2008).
Nakayama, I. et al. Enrichment of CLDN18-ARHGAP fusion gene in gastric cancers in young adults. Cancer Sci. 110, 1352–1363 (2019).
Singh, P., Toom, S. & Huang, Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J. Hematol. Oncol. 10, 105 (2017).
Tureci, O. et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann. Oncol. 30, 1487–1495 (2019).
Sahin, U. et al. Zolbetuximab combined with EOX as first-line therapy in advanced CLDN18.2+ gastric (G) and gastroesophageal junction (GEJ) adenocarcinoma: updated results from the FAST trial. Ann. Oncol. https://doi.org/10.1016/j.annonc.2021.02.005 (2021).
Moran, D., Maurus, D., Rohde, C. & Arozullah, A. 103P. Prevalence of CLDN18.2, HER2 and PD-L1 in gastric cancer samples. Ann. Oncol. 29, viii32 (2018).
Jiang, H. et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J. Natl Cancer Inst. 111, 409–418 (2019).
Zhan, X. et al. Phase I trial of claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. J. Clin. Oncol. 37, 2509–2509 (2019).
Shitara, K. et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018).
Shitara, K. et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 6, 1571–1580 (2020).
Shitara, K. et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J. Clin. Oncol. 38, 4537–4537 (2020).
Fuchs, C. S. et al. The association of molecular biomarkers with efficacy of pembrolizumab versus paclitaxel in patients with gastric cancer (GC) from KEYNOTE-061. J. Clin. Oncol. 38, 4512–4512 (2020).
Wyrwicz, L. S. et al. 1442P association of TMB using the foundation medicine companion diagnostic (F1CDx) with efficacy of first-line pembrolizumab (pembro) or pembro plus chemotherapy (pembro+chemo) versus chemo in patients with gastric cancer (gc) from KEYNOTE-062. Ann. Oncol. 31, S907–S908 (2020).
Boku, N. et al. LBA7_PR Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann. Oncol. 31, S1192 (2020).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Arlauckas, S. P. et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 9, eaal3604 (2017).
Kamada, T. et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116, 9999–10008 (2019).
Lo Russo, G. et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin. Cancer Res. 25, 989–999 (2019).
Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346–1358 (2020).
Hoff, S., Grünewald, S., Röse, L. & Zopf, D. 1198P. Immunomodulation by regorafenib alone and in combination with anti PD1 antibody on murine models of colorectal cancer. Ann. Oncol. https://doi.org/10.1093/annonc/mdx376.060 (2017).
Chen, C.-W. et al. FRI-471-regorafenib may enhance efficacy of anti-program cell death-1 therapy in hepatocellular carcinoma through modulation of macrophage polarization. J. Hepatol. 70, e605–e606 (2019).
Kato, Y. et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 14, e0212513 (2019).
Fukuoka, S. et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 38, 2053–2061 (2020).
Kawazoe, A. et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 21, 1057–1065 (2020).
Catenacci, D. V. T. et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): a phase II study evaluating an individualized treatment strategy for metastatic disease. Cancer Discov. 11, 308 (2021).
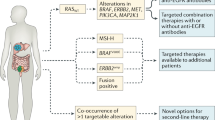
van Grieken, N. C. et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br. J. Cancer 108, 1495–1501 (2013).
Shinozaki-Ushiku, A. et al. The first case of gastric carcinoma with NTRK rearrangement: identification of a novel ATP1B-NTRK1 fusion. Gastric Cancer 23, 944–947 (2020).
Alese, O. B. et al. Anaplastic lymphoma kinase (ALK) gene alteration in signet ring cell carcinoma of the gastrointestinal tract. Ther. Adv. Med. Oncol. 7, 56–62 (2015).
Lee, J. et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer 119, 1627–1635 (2013).
Yuki, S. et al. The nationwide cancer genome screening project in Japan SCRUM-Japan GI-SCREEN: efficient identification of cancer genome alterations in advanced gastric cancer (GC). J. Clin. Oncol. 36, 4050–4050 (2018).
Nakamura, Y. et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 26, 1859–1864 (2020).
Acknowledgements
The work of Y.N. is supported by SCRUM-Japan Funds and a grant from the Japan Agency for Medical Research and Development (no. 19ck0106445h0002).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.N. has received research funding from Chugai Pharmaceutical, Genomedia, Guardant Health and Taiho Pharmaceutical. A.K. has received research funding from MSD, Ono, Sumitomo Dainippon and Taiho, and honoraria from Ono and Taiho. F.L. has received research funding from BMS, Iomedico and Zymeworks, and honoraria from Amgen, Astellas Pharma, AstraZeneca, Bayer, BioNTech, BMS, Eli Lilly, Elsevier, Excerpta Medica, Imedex, Infomedica, Iomedico, Medscape, MedUpdate, Merck Serono, MSD, Oncovis, Promedicis, Roche, SpringerNature, StreamedUp! and Zymeworks. Y.Y.J. has received institutional research funding from Bayer, Boehringer Ingelheim, BMS, Eli Lilly, Genentech/Roche, Merck and Roche, has acted as an advisor for AstraZeneca, Basilea Pharmaceutica, Bayer, BMS, Daiichi-Sankyo, Eli Lilly, Imugene, Merck, Merck Serono, Pfizer, Rgenix and Zymeworks, and holds stock options in Rgenix. K.S. has received research funding from Astellas, Chugai, Daiichi Sankyo, Eli Lilly, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Taiho, Medi Science and MSD, has acted as a consultant or advisor for AbbVie, Astellas, BMS, Eli Lilly, GSK, MSD, Novartis, Ono Pharmaceutical, Pfizer, Taiho and Takeda, and has received honoraria from AbbVie, Novartis and Yakult.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Clinical Oncology thanks L.-T. Chen, Y.-J. Bang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Rights and permissions
About this article
Cite this article
Nakamura, Y., Kawazoe, A., Lordick, F. et al. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol 18, 473–487 (2021). https://doi.org/10.1038/s41571-021-00492-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00492-2
This article is cited by
-
Efficacy and safety of Zolbetuximab plus chemotherapy for advanced CLDN18.2-positive gastric or gastro-oesophageal adenocarcinoma: a meta-analysis of randomized clinical trials
BMC Cancer (2024)
-
Clinical applications and perspectives of circulating tumor DNA in gastric cancer
Cancer Cell International (2024)
-
Claudin 18.2 as a novel therapeutic target
Nature Reviews Clinical Oncology (2024)
-
DNA mismatch repair deficiency and outcomes of patients with locally advanced gastric cancer treated with preoperative docetaxel, oxaliplatin, and S-1 plus surgery and postoperative S-1 or surgery plus postoperative S-1: a sub-analysis of the phase 3 PRODIGY trial
Gastric Cancer (2024)
-
The Molecular Landscape of Gastric Cancers for Novel Targeted Therapies from Real-World Genomic Profiling
Targeted Oncology (2024)