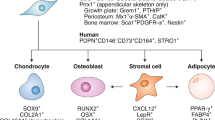
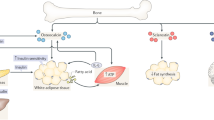
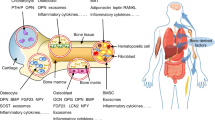
Abstract
Bone development and bone remodelling during adult life are highly anabolic processes requiring an adequate supply of oxygen and nutrients. Bone-forming osteoblasts and bone-resorbing osteoclasts interact closely to preserve bone mass and architecture and are often located close to blood vessels. Chondrocytes within the developing growth plate ensure that bone lengthening occurs before puberty, but these cells function in an avascular environment. With ageing, numerous bone marrow adipocytes appear, often with negative effects on bone properties. Many studies have now indicated that skeletal cells have specific metabolic profiles that correspond to the nutritional microenvironment and their stage-specific functions. These metabolic networks provide not only skeletal cells with sufficient energy, but also biosynthetic intermediates that are necessary for proliferation and extracellular matrix synthesis. Moreover, these metabolic pathways control redox homeostasis to avoid oxidative stress and safeguard cell survival. Finally, several intracellular metabolites regulate the activity of epigenetic enzymes and thus control the fate and function of skeletal cells. The metabolic profile of skeletal cells therefore not only reflects their cellular state, but can also drive cellular activity. Insight into skeletal cell metabolism will thus not only advance our understanding of skeletal development and homeostasis, but also of skeletal disorders, such as osteoarthritis, diabetic bone disease and bone malignancies.
Key points
-
Skeletal stem and progenitor cells display a high metabolic flexibility, and are likely to adapt to changing microenvironments.
-
Osteoblasts use glycolysis and fatty acid oxidation as major energy sources, whereas they use glutamine metabolism for biosynthesis and prevention of oxidative stress.
-
Bone marrow adipocytes release fatty acids upon systemic energy deficit to support osteoblast function.
-
Chondrocytes are well-adapted to their hypoxic and fatty acid-scarce microenvironment, as they rely on glycolysis for bioenergetics and glutamine metabolism for biosynthesis, redox balance and epigenetic regulation.
-
Osteoclast differentiation depends on oxidative phosphorylation, primarily supplied by glucose and fatty acid oxidation.
-
Metabolic disturbance is linked to skeletal cell dysfunction during bone pathology, and bone-metastatic and leukaemic cells hijack skeletal cell metabolism to support their tumorigenic spread.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peck, W. A., Birge, S. J. Jr. & Fedak, S. A. Bone cells: biochemical and biological studies after enzymatic isolation. Science 146, 1476–1477 (1964).
Otte, P. Basic cell metabolism of articular cartilage. Manometric studies. Z. Rheumatol. 50, 304–312 (1991).
Borle, A. B., Nichols, N. & Nichols, G. Jr. Metabolic studies of bone in vitro. I. Normal bone. J. Biol. Chem. 235, 1206–1210 (1960).
Stegen, S. & Carmeliet, G. The skeletal vascular system – breathing life into bone tissue. Bone 115, 50–58 (2018).
Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 (2011).
Ikeda, K. & Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 159, 1–8 (2016).
Jacome-Galarza, C. E. et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 568, 541–545 (2019).
Kurenkova, A. D., Medvedeva, E. V., Newton, P. T. & Chagin, A. S. Niches for skeletal stem cells of mesenchymal origin. Front. Cell Dev. Biol. 8, 592 (2020).
Roberts, S. J., van Gastel, N., Carmeliet, G. & Luyten, F. P. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 70, 10–18 (2015).
Ambrosi, T. H., Longaker, M. T. & Chan, C. K. F. A revised perspective of skeletal stem cell biology. Front. Cell Dev. Biol. 7, 189 (2019).
Matsushita, Y., Ono, W. & Ono, N. Skeletal stem cells for bone development and repair: diversity matters. Curr. Osteoporos. Rep. 18, 189–198 (2020).
Feng, H. et al. Skeletal stem cells: origins, definitions, and functions in bone development and disease. Life Med. 1, 276–293 (2022).
Robling, A. G. & Bonewald, L. F. The osteocyte: new insights. Annu. Rev. Physiol. 82, 485–506 (2020).
Delgado-Calle, J. & Bellido, T. The osteocyte as a signaling cell. Physiol. Rev. 102, 379–410 (2022).
Dobnig, H. & Turner, R. T. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136, 3632–3638 (1995).
Long, F. & Ornitz, D. M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 5, a008334 (2013).
Hallett, S. A., Ono, W. & Ono, N. The hypertrophic chondrocyte: to be or not to be. Histol. Histopathol. 36, 1021–1036 (2021).
Goldring, M. B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 4, 269–285 (2012).
Zhao, Z. et al. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J. Cell Mol. Med. 24, 5408–5419 (2020).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020).
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the Intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Martinez-Reyes, I. & Chandel, N. S. Cancer metabolism: looking forward. Nat. Rev. Cancer 21, 669–680 (2021).
Intlekofer, A. M. & Finley, L. W. S. Metabolic signatures of cancer cells and stem cells. Nat. Metab. 1, 177–188 (2019).
Couasnay, G., Madel, M. B., Lim, J., Lee, B. & Elefteriou, F. Sites of Cre-recombinase activity in mouse lines targeting skeletal cells. J. Bone Miner. Res. 36, 1661–1679 (2021).
Tournaire, G. et al. Skeletal progenitors preserve proliferation and self-renewal upon inhibition of mitochondrial respiration by rerouting the TCA cycle. Cell Rep. 40, 111105 (2022).
Stegen, S. & Carmeliet, G. Hypoxia, hypoxia-inducible transcription factors and oxygen-sensing prolyl hydroxylases in bone development and homeostasis. Curr. Opin. Nephrol. Hypertens. 28, 328–335 (2019).
Lee, S. Y., Abel, E. D. & Long, F. Glucose metabolism induced by Bmp signaling is essential for murine skeletal development. Nat. Commun. 9, 4831 (2018).
Jeoung, N. H. Pyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancers. Diabetes Metab. J. 39, 188–197 (2015).
Heinemann-Yerushalmi, L. et al. BCKDK regulates the TCA cycle through PDC in the absence of PDK family during embryonic development. Dev. Cell 56, 1182–1194.e6 (2021).
van Gastel, N. et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 579, 111–117 (2020).
Hu, G. et al. The amino acid sensor Eif2ak4/GCN2 is required for proliferation of osteoblast progenitors in mice. J. Bone Miner. Res. 35, 2004–2014 (2020).
Devignes, C. S., Carmeliet, G. & Stegen, S. Amino acid metabolism in skeletal cells. Bone Rep. 17, 101620 (2022).
Stegen, S. et al. HIF-1ɑ promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 23, 265–279 (2016).
Stegen, S. et al. Glutamine metabolism controls chondrocyte identity and function. Dev. Cell 53, 530–544.e8 (2020).
Yu, Y. et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 29, 966–978.e4 (2019).
Solidum, J. G. N., Jeong, Y., Heralde, F. 3rd & Park, D. Differential regulation of skeletal stem/progenitor cells in distinct skeletal compartments. Front. Physiol. 14, 1137063 (2023).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Loopmans, S., Stockmans, I., Carmeliet, G. & Stegen, S. Isolation and in vitro characterization of murine young-adult long bone skeletal progenitors. Front. Endocrinol. 13, 930358 (2022).
Stegen, S. et al. Adequate hypoxia inducible factor 1ɑ signaling is indispensable for bone regeneration. Bone 87, 176–186 (2016).
Kristensen, H. B., Andersen, T. L., Marcussen, N., Rolighed, L. & Delaisse, J. M. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J. Bone Miner. Res. 28, 574–585 (2013).
Wei, J. et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell 161, 1576–1591 (2015).
Li, Z. et al. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology 157, 4094–4103 (2016).
Lee, W. C., Ji, X., Nissim, I. & Long, F. Malic enzyme couples mitochondria with aerobic glycolysis in osteoblasts. Cell Rep. 32, 108108 (2020).
Broeks, M. H., van Karnebeek, C. D. M., Wanders, R. J. A., Jans, J. J. M. & Verhoeven-Duif, N. M. Inborn disorders of the malate aspartate shuttle. J. Inherit. Metab. Dis. 44, 792–808 (2021).
Chen, C. T., Shih, Y. R., Kuo, T. K., Lee, O. K. & Wei, Y. H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cell 26, 960–968 (2008).
Kim, S. P. et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight 2, e92704 (2017).
Bartelt, A. et al. Quantification of bone fatty acid metabolism and its regulation by adipocyte lipoprotein lipase. Int. J. Mol. Sci. 18, 1264 (2017).
Stegen, S. et al. Glutamine metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male mice. J. Bone Miner. Res. 36, 604–616 (2021).
Sharma, D., Yu, Y., Shen, L., Zhang, G. F. & Karner, C. M. SLC1A5 provides glutamine and asparagine necessary for bone development in mice. Elife 10, e71595 (2021).
Hu, G. Glutathione limits RUNX2 oxidation and degradation to regulate bone formation. JCI Insight 8, e166888 (2023).
Berger, J. M. et al. Mediation of the acute stress response by the skeleton. Cell Metab. 30, 890–902 e898 (2019).
Shen, L. et al. SLC38A2 provides proline to fulfill unique synthetic demands arising during osteoblast differentiation and bone formation. Elife 11, e76963 (2022).
Shen, L., Yu, Y. & Karner, C. M. SLC38A2 provides proline and alanine to regulate postnatal bone mass accrual in mice. Front. Physiol. 13, 992679 (2022).
Jeon, Y. G., Kim, Y. Y., Lee, G. & Kim, J. B. Physiological and pathological roles of lipogenesis. Nat. Metab. 5, 735–759 (2023).
Dickens, F. The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem. J. 35, 1011–1023 (1941).
Hu, Y. Y., Rawal, A. & Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl Acad. Sci. USA 107, 22425–22429 (2010).
Davies, E. et al. Citrate bridges between mineral platelets in bone. Proc. Natl Acad. Sci. USA 111, E1354–E1363 (2014).
Pajor, A. M. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflug. Arch. 466, 119–130 (2014).
Dirckx, N. et al. A specialized metabolic pathway partitions citrate in hydroxyapatite to impact mineralization of bones and teeth. Proc. Natl Acad. Sci. USA 119, e2212178119 (2022).
Cosman, F., Nieves, J. W. & Dempster, D. W. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J. Bone Miner. Res. 32, 198–202 (2017).
Jilka, R. L. et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest. 104, 439–446 (1999).
Kim, S. W. et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. 27, 2075–2084 (2012).
Wu, X. et al. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 7, 571–580 (2010).
Esen, E., Lee, S. Y., Wice, B. M. & Long, F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J. Bone Miner. Res. 30, 1959–1968 (2015).
Alekos, N. S. et al. Mitochondrial β-oxidation of adipose-derived fatty acids by osteoblasts fuels parathyroid hormone-induced bone formation. JCI Insight 8, e165604 (2023).
Maridas, D. E. et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. FASEB J. 33, 2885–2898 (2019).
Esen, E. et al. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 17, 745–755 (2013).
Chen, H. et al. Increased glycolysis mediates Wnt7b-induced bone formation. FASEB J. 33, 7810–7821 (2019).
Karner, C. M., Esen, E., Okunade, A. L., Patterson, B. W. & Long, F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J. Clin. Invest. 125, 551–562 (2015).
Frey, J. L. et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol. Cell Biol. 35, 1979–1991 (2015).
Frey, J. L., Kim, S. P., Li, Z., Wolfgang, M. J. & Riddle, R. C. Beta-catenin directs long-chain fatty acid catabolism in the osteoblasts of male mice. Endocrinology 159, 272–284 (2018).
van Gastel, N. & Carmeliet, G. Metabolic regulation of skeletal cell fate and function in physiology and disease. Nat. Metab. 3, 11–20 (2021).
Regan, J. N. et al. Up-regulation of glycolytic metabolism is required for HIF1ɑ-driven bone formation. Proc. Natl Acad. Sci. USA 111, 8673–8678 (2014).
Dirckx, N. et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J. Clin. Invest. 128, 1087–1105 (2018).
Scheller, E. L., Cawthorn, W. P., Burr, A. A., Horowitz, M. C. & MacDougald, O. A. Marrow adipose tissue: trimming the fat. Trends Endocrinol. Metab. 27, 392–403 (2016).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Zhong, L. et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife 9, e54695 (2020).
Hu, Y. et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 22, e52481 (2021).
Yu, W. et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J. Clin. Invest 131, e140214 (2021).
Zhong, L. et al. Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone. Elife 12, e82112 (2023).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 (2017).
Tencerova, M. et al. Metabolic programming determines the lineage-differentiation fate of murine bone marrow stromal progenitor cells. Bone Res. 7, 35 (2019).
Li, Z. et al. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. Elife 11, e78946 (2022).
Suchacki, K. J. et al. Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat. Commun. 11, 3097 (2020).
Scheller, E. L. et al. Bone marrow adipocytes resist lipolysis and remodeling in response to β-adrenergic stimulation. Bone 118, 32–41 (2019).
Schipani, E. et al. Hypoxia in cartilage: HIF-1ɑ is essential for chondrocyte growth arrest and survival. Genes. Dev. 15, 2865–2876 (2001).
Maes, C. et al. VEGF-independent cell-autonomous functions of HIF-1ɑ regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J. Bone Miner. Res. 27, 596–609 (2012).
Yao, Q. et al. Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev. Cell 49, 748–763.e7 (2019).
Stegen, S. et al. HIF-1ɑ metabolically controls collagen synthesis and modification in chondrocytes. Nature 565, 511–515 (2019).
Lin, C. et al. Impaired mitochondrial oxidative metabolism in skeletal progenitor cells leads to musculoskeletal disintegration. Nat. Commun. 13, 6869 (2022).
Zhang, F. et al. An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption. Nature 622, 834–841 (2023).
Stegen, S. et al. De novo serine synthesis regulates chondrocyte proliferation during bone development and repair. Bone Res. 10, 14 (2022).
Stegen, S., van Gastel, N. & Carmeliet, G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone 70, 19–27 (2015).
Godman, G. C. & Porter, K. R. Chondrogenesis, studied with the electron microscope. J. Biophys. Biochem. Cytol. 8, 719–760 (1960).
Pathmanapan, S. et al. Mutant IDH regulates glycogen metabolism from early cartilage development to malignant chondrosarcoma formation. Cell Rep. 42, 112578 (2023).
Torzilli, P. A., Grande, D. A. & Arduino, J. M. Diffusive properties of immature articular cartilage. J. Biomed. Mater. Res. 40, 132–138 (1998).
Prendeville, H. & Lynch, L. Diet, lipids, and antitumor immunity. Cell Mol. Immunol. 19, 432–444 (2022).
Kikuchi, M. et al. Crucial role of Elovl6 in chondrocyte growth and differentiation during growth plate development in mice. PLoS ONE 11, e0159375 (2016).
Tsushima, H. et al. Intracellular biosynthesis of lipids and cholesterol by Scap and Insig in mesenchymal cells regulates long bone growth and chondrocyte homeostasis. Development 145, dev162396 (2018).
Park-Min, K. H. et al. Inhibition of osteoclastogenesis and inflammatory bone resorption by targeting BET proteins and epigenetic regulation. Nat. Commun. 5, 5418 (2014).
Ishii, K. A. et al. Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 15, 259–266 (2009).
Bae, S. et al. MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRɑ. J. Clin. Invest. 127, 2555–2568 (2017).
Zhang, Y. et al. PGC1β organizes the osteoclast cytoskeleton by mitochondrial biogenesis and activation. J. Bone Miner. Res. 33, 1114–1125 (2018).
Jin, Z., Wei, W., Yang, M., Du, Y. & Wan, Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 20, 483–498 (2014).
Kushwaha, P. et al. Mitochondrial fatty acid β-oxidation is important for normal osteoclast formation in growing female mice. Front. Physiol. 13, 997358 (2022).
Kim, H. N. et al. Estrogens decrease osteoclast number by attenuating mitochondria oxidative phosphorylation and ATP production in early osteoclast precursors. Sci. Rep. 10, 11933 (2020).
Nishikawa, K. et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat. Med. 21, 281–287 (2015).
Kurotaki, D., Yoshida, H. & Tamura, T. Epigenetic and transcriptional regulation of osteoclast differentiation. Bone 138, 115471 (2020).
Rohatgi, N. et al. BAP1 promotes osteoclast function by metabolic reprogramming. Nat. Commun. 14, 5923 (2023).
Li, B. et al. Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. FASEB J. 34, 11058–11067 (2020).
Indo, Y. et al. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 28, 2392–2399 (2013).
Arnett, T. R. & Orriss, I. R. Metabolic properties of the osteoclast. Bone 115, 25–30 (2018).
Stegen, S., Moermans, K., Stockmans, I., Thienpont, B. & Carmeliet, G. The serine synthesis pathway drives osteoclast differentiation through epigenetic regulation of NFATc1 expression. Nat. Metab. 6, 141–152 (2024).
Ozaki, K. et al. The L-type amino acid transporter LAT1 inhibits osteoclastogenesis and maintains bone homeostasis through the mTORC1 pathway. Sci. Signal 12, eaaw3921 (2019).
Go, M. et al. BCAT1 promotes osteoclast maturation by regulating branched-chain amino acid metabolism. Exp. Mol. Med. 54, 825–833 (2022).
Pereira, M. et al. A trans-eQTL network regulates osteoclast multinucleation and bone mass. Elife 9, e55549 (2020).
Brunner, J. S. et al. Environmental arginine controls multinuclear giant cell metabolism and formation. Nat. Commun. 11, 431 (2020).
Cao, S. et al. L-arginine metabolism inhibits arthritis and inflammatory bone loss. Ann. Rheum. Dis. 83, 72–87 (2023).
Mobasheri, A. et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13, 302–311 (2017).
Zheng, L., Zhang, Z., Sheng, P. & Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 66, 101249 (2021).
Arra, M. et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 11, 3427 (2020).
Li, K. et al. Impaired glucose metabolism underlies articular cartilage degeneration in osteoarthritis. FASEB J. 36, e22377 (2022).
Wang, C. et al. Deletion of Glut1 in early postnatal cartilage reprograms chondrocytes toward enhanced glutamine oxidation. Bone Res. 9, 38 (2021).
Choi, W. S. et al. The CH25H–CYP7B1–RORɑ axis of cholesterol metabolism regulates osteoarthritis. Nature 566, 254–258 (2019).
Ratneswaran, A. et al. Peroxisome proliferator-activated receptor δ promotes the progression of posttraumatic osteoarthritis in a mouse model. Arthritis Rheumatol. 67, 454–464 (2015).
Choi, W. S. et al. Critical role for arginase II in osteoarthritis pathogenesis. Ann. Rheum. Dis. 78, 421–428 (2019).
Napoli, N. et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 13, 208–219 (2017).
Yamamoto, M. & Sugimoto, T. Advanced glycation end products, diabetes, and bone strength. Curr. Osteoporos. Rep. 14, 320–326 (2016).
Ji, X. et al. Genetic activation of glycolysis in osteoblasts preserves bone mass in type I diabetes. Cell Chem. Biol. 30, 1053–1063.e5 (2023).
Song, F. et al. Osteoblast-intrinsic defect in glucose metabolism impairs bone formation in type II diabetic male mice. Elife 12, e85714 (2023).
Bergers, G. & Fendt, S. M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug. Discov. 21, 141–162 (2022).
Makhoul, I., Montgomery, C. O., Gaddy, D. & Suva, L. J. The best of both worlds – managing the cancer, saving the bone. Nat. Rev. Endocrinol. 12, 29–42 (2016).
Dupuy, F. et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 22, 577–589 (2015).
Whitburn, J. et al. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci. Adv. 8, eabf9096 (2022).
Stincone, A. et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 90, 927–963 (2015).
Tirado, H. A., Balasundaram, N., Laaouimir, L., Erdem, A. & van Gastel, N. Metabolic crosstalk between stromal and malignant cells in the bone marrow niche. Bone Rep. 18, 101669 (2023).
He, X. et al. Bone marrow niche ATP levels determine leukemia-initiating cell activity via P2X7 in leukemic models. J. Clin. Invest 131, e140242 (2021).
Shafat, M. S. et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood 129, 1320–1332 (2017).
Vilaplana-Lopera, N. et al. Crosstalk between AML and stromal cells triggers acetate secretion through the metabolic rewiring of stromal cells. Elife 11, e75908 (2022).
Galan-Diez, M. et al. Subversion of serotonin receptor signaling in osteoblasts by kynurenine drives acute myeloid leukemia. Cancer Discov. 12, 1106–1127 (2022).
van Gastel, N. et al. Induction of a timed metabolic collapse to overcome cancer chemoresistance. Cell Metab. 32, 391–403.e6 (2020).
Zhang, W. et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 14, 276–286 (2012).
Panaroni, C. et al. Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood 139, 876–888 (2022).
Acknowledgements
The authors thank Fonds voor Wetenschappelijk Onderzoek-Flanders for funding: EOS-G0F8218N, G0B3418N, G0C5120, G071321N, Hercules-I013518N.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Martina Rauner, Ryan Riddle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stegen, S., Carmeliet, G. Metabolic regulation of skeletal cell fate and function. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-00969-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-024-00969-x