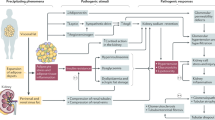
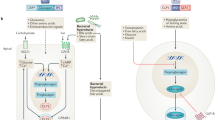
Abstract
Obesity is strongly associated with the development of diabetes mellitus and chronic kidney disease (CKD), but there is evidence for a bidirectional relationship wherein the kidney also acts as a key regulator of body weight. In this Review, we highlight the mechanisms implicated in obesity-related CKD, and outline how the kidney might modulate feeding and body weight through a growth differentiation factor 15-dependent kidney–brain axis. The favourable effects of bariatric surgery on kidney function are discussed, and medical therapies designed for the treatment of diabetes mellitus that lower body weight and preserve kidney function independent of glycaemic lowering, including sodium–glucose cotransporter 2 inhibitors, incretin-based therapies and metformin, are also reviewed. In summary, we propose that kidney function and body weight are related in a bidirectional fashion, and that this interrelationship affects human health and disease.
Key points
-
Obesity alters kidney function through multiple direct and indirect mechanisms.
-
Sodium–glucose cotransporter 2 inhibitors (SGLT2is) and glucagon-like peptide 1 receptor agonists (GLP1RAs) both promote weight loss; they also stabilize kidney function through mechanisms that are independent of their effects on weight.
-
SGLT2is induce a metabolic shift towards ketogenesis, which is likely to account for sustained weight loss.
-
The beneficial effects of GLP1RAs on the kidney are not solely explained by weight loss or improvement in glycaemic control.
-
Several GLP1R dual or polyagonist agents are under evaluation with the potential to achieve greater weight loss than GLP1RAs alone.
-
Metformin increases levels of growth differentiation factor 15 (GDF15) in the kidney and circulation to activate glial cell line-derived neurotrophic factor family receptor ɑ-like (GFRAL) in the area postrema to reduce body weight.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Kovesdy, C. P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. 12, 7–11 (2022).
Eckel, R. H. & Cornier, M. A. Update on the NCEP ATP-III emerging cardiometabolic risk factors. BMC Med. 12, 115 (2014).
Ndumele, C. E. et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation 148, 1606–1635 (2023).
Tchernof, A. & Després, J. P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93, 359–404 (2013).
Ye, C. et al. Causal associations of obesity with chronic kidney disease and arterial stiffness: a mendelian randomization study. J. Clin. Endocrinol. Metab. 107, e825–e835 (2022).
Chang, A. R. et al. Adiposity and risk of decline in glomerular filtration rate: meta-analysis of individual participant data in a global consortium. BMJ 364, k5301 (2019). This meta-analysis demonstrates that increased BMI is an independent risk factor for GFR decline and death in the general population and those with CKD.
Hsu, C. Y., McCulloch, C. E., Iribarren, C., Darbinian, J. & Go, A. S. Body mass index and risk for end-stage renal disease. Ann. Intern. Med. 144, 21–28 (2006).
Zhang, S. Y. et al. Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight. Cell Metab. 35, 875–886.e5 (2023). This study demonstrates that metformin mediates weight loss through a kidney GDF15-dependent area postrema axis.
Weisinger, J. R., Kempson, R. L., Eldridge, F. L. & Swenson, R. S. The nephrotic syndrome: a complication of massive obesity. Ann. Intern. Med. 81, 440–447 (1974).
D’Agati, V. D., Fogo, A. B., Bruijn, J. A. & Jennette, J. C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am. J. Kidney Dis. 43, 368–382 (2004).
Kambham, N., Markowitz, G. S., Valeri, A. M., Lin, J. & D’Agati, V. D. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 59, 1498–1509 (2001).
Chen, H. M. et al. Podocyte lesions in patients with obesity-related glomerulopathy. Am. J. Kidney Dis. 48, 772–779 (2006).
Chen, H. M. et al. Obesity-related glomerulopathy in China: a case series of 90 patients. Am. J. Kidney Dis. 52, 58–65 (2008).
D’Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12, 453–471 (2016).
Praga, M. et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol. Dial. Transpl. 16, 1790–1798 (2001).
Serra, A. et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 73, 947–955 (2008).
Griffin, K. A., Kramer, H. & Bidani, A. K. Adverse renal consequences of obesity. Am. J. Physiol. Ren. Physiol. 294, F685–F696 (2008).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 116, 991–1006 (2015).
Schorr, U., Blaschke, K., Turan, S., Distler, A. & Sharma, A. M. Relationship between angiotensinogen, leptin and blood pressure levels in young normotensive men. J. Hypertens. 16, 1475–1480 (1998).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 15, 367–385 (2019).
Goodfriend, T. L., Ball, D. L., Egan, B. M., Campbell, W. B. & Nithipatikom, K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension 43, 358–363 (2004).
Jeon, J. H. et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 22, 1502–1511 (2008).
Mallamaci, F. et al. ACE inhibition is renoprotective among obese patients with proteinuria. J. Am. Soc. Nephrol. 22, 1122–1128 (2011).
Kim, S. et al. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J. Biomed. Biotechnol. 2006, 27012 (2006).
Henegar, J. R. et al. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am. J. Hypertens. 27, 1285–1292 (2014).
Xu, X., Huang, X., Zhang, L., Qin, Z. & Hua, F. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-κB/NLRP3 inflammation pathway. BMC Nephrol. 22, 218 (2021).
Hall, J. E. et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 285, 17271–17276 (2010).
Mansukhani, M. P., Wang, S. & Somers, V. K. Chemoreflex physiology and implications for sleep apnoea: insights from studies in humans. Exp. Physiol. 100, 130–135 (2015).
Hall, M. E. et al. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renovasc Dis. 7, 75–88 (2014).
Eddy, A. A. & Fogo, A. B. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J. Am. Soc. Nephrol. 17, 2999–3012 (2006).
Benomar, Y. et al. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes 62, 102–114 (2013).
Yano, Y. et al. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J. Clin. Hypertens. 9, 775–782 (2007).
Sharma, K. et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 118, 1645–1656 (2008).
Fang, F. et al. Deletion of the gene for adiponectin accelerates diabetic nephropathy in the Ins2+/C96Y mouse. Diabetologia 58, 1668–1678 (2015).
Ohashi, K. et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler. Thromb. Vasc. Biol. 27, 1910–1917 (2007).
Briffa, J. F., McAinch, A. J., Poronnik, P. & Hryciw, D. H. Adipokines as a link between obesity and chronic kidney disease. Am. J. Physiol. Ren. Physiol. 305, F1629–F1636 (2013).
Aizawa-Abe, M. et al. Pathophysiological role of leptin in obesity-related hypertension. J. Clin. Invest. 105, 1243–1252 (2000).
Huby, A. C. et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132, 2134–2145 (2015).
Belin de Chantemèle, E. J., Mintz, J. D., Rainey, W. E. & Stepp, D. W. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension 58, 271–279 (2011).
de Vries, A. P. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2, 417–426 (2014).
Zhao, J. et al. CD36-mediated lipid accumulation and activation of NLRP3 inflammasome lead to podocyte injury in obesity-related glomerulopathy. Mediators Inflamm. 2019, 3172647 (2019).
Shahzad, K. et al. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 102, 766–779 (2022).
Hou, Y. et al. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem. Biophys. Res. Commun. 521, 791–798 (2020).
Qiu, Y. Y. & Tang, L. Q. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol. Res. 114, 251–264 (2016).
Ke, B., Shen, W., Fang, X. & Wu, Q. The NLPR3 inflammasome and obesity-related kidney disease. J. Cell Mol. Med. 22, 16–24 (2018).
Rampanelli, E. et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Sci. Rep. 7, 2861 (2017).
Yamamoto, T. et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 28, 1534–1551 (2017).
Bakker, P. J. et al. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int. 85, 1112–1122 (2014).
Chiazza, F. et al. Targeting the NLRP3 inflammasome to reduce diet-induced metabolic abnormalities in mice. Mol. Med. 21, 1025–1037 (2016).
The Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35, 731–737 (2012).
Seifarth, C., Schehler, B. & Schneider, H. J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 121, 27–31 (2013).
Sjöström, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Alexander, J. W., Goodman, H. R., Hawver, L. R. & Cardi, M. A. Improvement and stabilization of chronic kidney disease after gastric bypass. Surg. Obes. Relat. Dis. 5, 237–241 (2009).
Bilha, S. C. et al. The effects of bariatric surgery on renal outcomes: a systematic review and meta-analysis. Obes. Surg. 28, 3815–3833 (2018).
Inge, T. H. et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N. Engl. J. Med. 374, 113–123 (2016).
Schiavon, C. A. et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation 137, 1132–1142 (2018).
Scheurlen, K. M. et al. Metabolic surgery improves renal injury independent of weight loss: a meta-analysis. Surg. Obes. Relat. Dis. 15, 1006–1020 (2019).
MacLaughlin, H. L. et al. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3-4 CKD: a randomized controlled pilot study. Am. J. Kidney Dis. 64, 660–663 (2014).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Moritz, E. et al. Renal sinus fat is expanded in patients with obesity and/or hypertension and reduced by bariatric surgery associated with hypertension remission. Metabolites 12, 617 (2022).
Ricci, M. A. et al. Morbid obesity and hypertension: the role of perirenal fat. J. Clin. Hypertens. 20, 1430–1437 (2018).
Docherty, N. G. & le Roux, C. W. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat. Rev. Nephrol. 16, 709–720 (2020).
Ghezzi, C., Loo, D. D. F. & Wright, E. M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 61, 2087–2097 (2018).
Shyangdan, D. S., Uthman, O. A. & Waugh, N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 6, e009417 (2016).
Herrington, W. G. et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2023).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Cherney, D. Z. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
van Bommel, E. J. M. et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 97, 202–212 (2020).
Körner, A., Eklöf, A. C., Celsi, G. & Aperia, A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 43, 629–633 (1994).
Vallon, V. & Thomson, S. C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 16, 317–336 (2020).
Ferrannini, E., Mark, M. & Mayoux, E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 39, 1108–1114 (2016).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Mayne, K. J. et al. Effects of empagliflozin on fluid overload, weight and blood pressure in chronic kidney disease. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.0000000000000271 (2023).
Yau, K., Dharia, A., Alrowiyti, I. & Cherney, D. Z. I. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int. Rep. 7, 1463–1476 (2022).
Thomas, M. C. & Cherney, D. Z. I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 61, 2098–2107 (2018).
Horie, I. et al. Increased sugar intake as a form of compensatory hyperphagia in patients with type 2 diabetes under dapagliflozin treatment. Diabetes Res. Clin. Pract. 135, 178–184 (2018).
Ferrannini, G. et al. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 38, 1730–1735 (2015).
Rajeev, S. P. et al. No evidence of compensatory changes in energy balance, despite reductions in body weight and liver fat, during dapagliflozin treatment in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, cross-over trial (ENERGIZE). Diabetes Obes. Metab. 25, 3621–3631 (2023).
Sargeant, J. A. et al. The effects of empagliflozin, dietary energy restriction, or both on appetite-regulatory gut peptides in individuals with type 2 diabetes and overweight or obesity: the SEESAW randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 24, 1509–1521 (2022).
Cefalu, W. T. Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition. J. Clin. Invest. 124, 485–487 (2014).
Ansary, T. M., Nakano, D. & Nishiyama, A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int. J. Mol. Sci. 20, 629 (2019).
Marton, A. et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat. Rev. Nephrol. 17, 65–77 (2021).
Yasui, A. et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 9, 863–871 (2018).
Wild, J. et al. Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol. 232, e13628 (2021).
Kitada, K. et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J. Clin. Invest. 127, 1944–1959 (2017).
Cherney, D. Z. I. et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 93, 231–244 (2018).
DeFronzo, R. A., Reeves, W. B. & Awad, A. S. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17, 319–334 (2021).
Xu, L. et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine 20, 137–149 (2017).
Tomita, I. et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 32, 404–419.e6 (2020). This study demonstrates that SGLT2 inhibitors increase ketone bodies to correct mTORC1 overactivation to mediate kidney protection in DKD.
Holst, J. J., Orskov, C., Nielsen, O. V. & Schwartz, T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 211, 169–174 (1987).
Cherney, D. Z. I., Udell, J. A. & Drucker, D. J. Cardiorenal mechanisms of action of glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors. Med 2, 1203–1230 (2021).
Kristensen, S. L. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7, 776–785 (2019).
Shi, Q. et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet 399, 259–269 (2022).
Sisley, S. et al. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Invest. 124, 2456–2463 (2014).
Heiss, C. N. et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 35, 109163 (2021).
Drucker, D. J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 57, 101351 (2022).
Turton, M. D. et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 (1996).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Fortin, S. M. et al. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Sci. Transl. Med. 12, eaay8071 (2020).
Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol. Metab. 46, 101102 (2021).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Yau, K., Odutayo, A., Dash, S. & Cherney, D. Z. I. Biology and clinical use of glucagon-like-peptide-1 receptor agonists in vascular protection. Can. J. Cardiol. 39, 1816–1838 (2023).
Kawanami, D. & Takashi, Y. GLP-1 receptor agonists in diabetic kidney disease: from clinical outcomes to mechanisms. Front. Pharmacol. 11, 967 (2020).
Górriz, J. L. et al. GLP-1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J. Clin. Med. 9, 947 (2020).
Gutzwiller, J. P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004).
Tonneijck, L. et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia 59, 1412–1421 (2016).
Rossing, P. et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol. Dial. Transpl. 38, 2041–2051 (2023).
Willard, F. S. et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 5, e140532 (2020).
Heerspink, H. J. L. et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 10, 774–785 (2022).
Sánchez-Garrido, M. A. et al. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia 60, 1851–1861 (2017).
Jastreboff, A. M. et al. Triple-hormone-receptor agonist retatrutide for obesity – a phase 2 trial. N. Engl. J. Med. 389, 514–526 (2023).
Kaneko, K. et al. Gut-derived GIP activates central Rap1 to impair neural leptin sensitivity during overnutrition. J. Clin. Invest. 129, 3786–3791 (2019).
Killion, E. A. et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci. Transl. Med. 10, eaat3392 (2018).
Zhang, Q. et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 33, 833–844.e5 (2021).
Adriaenssens, A. E. et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 30, 987–996.e6 (2019).
Quiñones, M. et al. Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance. Mol. Metab. 4, 961–970 (2015).
Lu, S. C. et al. GIPR antagonist antibodies conjugated to GLP-1 peptide are bispecific molecules that decrease weight in obese mice and monkeys. Cell Rep. Med. 2, 100263 (2021).
Boyle, C. N., Lutz, T. A. & Le Foll, C. Amylin – its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol. Metab. 8, 203–210 (2018).
Enebo, L. B. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet 397, 1736–1748 (2021).
Chen, W., Binbin, G., Lidan, S., Qiang, Z. & Jing, H. Evolution of peptide YY analogs for the management of type 2 diabetes and obesity. Bioorg. Chem. 140, 106808 (2023).
Hall, M. E., Jordan, J. H., Juncos, L. A., Hundley, W. G. & Hall, J. E. BOLD magnetic resonance imaging in nephrology. Int. J. Nephrol. Renovasc Dis. 11, 103–112 (2018).
Miller, R. A. et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260 (2013).
Day, E. A. et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 1, 1202–1208 (2019).
Coll, A. P. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020). This study demonstrated that metformin increases circulating GDF15, which is necessary for its effects on energy balance and body weight.
Bootcov, M. R. et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proc. Natl Acad. Sci. USA 94, 11514–11519 (1997).
Lawton, L. N. et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene 203, 17–26 (1997).
Böttner, M., Suter-Crazzolara, C., Schober, A. & Unsicker, K. Expression of a novel member of the TGF-β superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 297, 103–110 (1999).
Patel, S. et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 29, 707–718.e8 (2019).
Wang, D. et al. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 17, 592–607 (2021).
Hsu, J. Y. et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550, 255–259 (2017).
Yang, L. et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 23, 1158–1166 (2017).
Mullican, S. E. et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 23, 1150–1157 (2017).
Emmerson, P. J. et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 23, 1215–1219 (2017).
Wang, D. et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 619, 143–150 (2023).
Xiong, Y. et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci. Transl. Med. 9, eaan8732 (2017).
Sabatini, P. V. et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl Acad. Sci. USA 118, e2021357118 (2021).
Blundell, J. et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 19, 1242–1251 (2017).
Frikke-Schmidt, H. et al. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol. Metab. 21, 13–21 (2019).
Chrysovergis, K. et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int. J. Obes. 38, 1555–1564 (2014).
Gerstein, H. C. et al. Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care 40, 280–283 (2017).
Kwon, S. et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 43, 948–955 (2020).
Lee, M. et al. Phosphorylation of acetyl-CoA carboxylase by AMPK reduces renal fibrosis and is essential for the anti-fibrotic effect of metformin. J. Am. Soc. Nephrol. 29, 2326–2336 (2018).
Han, Y. C. et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 12, 925 (2021).
Christensen, M. et al. Renoprotective effects of metformin are independent of organic cation transporters 1 & 2 and AMP-activated protein kinase in the kidney. Sci. Rep. 6, 35952 (2016).
Mazagova, M. et al. Genetic deletion of growth differentiation factor 15 augments renal damage in both type 1 and type 2 models of diabetes. Am. J. Physiol. Ren. Physiol. 305, F1249–F1264 (2013).
Valiño-Rivas, L. et al. Growth differentiation factor-15 preserves Klotho expression in acute kidney injury and kidney fibrosis. Kidney Int. 101, 1200–1215 (2022).
Zhou, Z. et al. Circulating GDF-15 in relation to the progression and prognosis of chronic kidney disease: a systematic review and dose-response meta-analysis. Eur. J. Intern. Med. 110, 77–85 (2023).
Nair, V. et al. Growth differentiation factor-15 and risk of CKD progression. J. Am. Soc. Nephrol. 28, 2233–2240 (2017).
Breit, S. N., Brown, D. A. & Tsai, V. W. W. GDF15 analogs as obesity therapeutics. Cell Metab. 35, 227–228 (2023).
Connelly, P. W. et al. Growth differentiation factor 15 is decreased by kidney transplantation. Clin. Biochem. 73, 57–61 (2019).
Seyfried, F. et al. Weight loss from caloric restriction vs Roux-en-Y gastric bypass surgery differentially regulates systemic and portal vein GDF15 levels in obese Zucker fatty rats. Physiol. Behav. 240, 113534 (2021).
Dolo, P. R. et al. Effect of sleeve gastrectomy on plasma growth differentiation factor-15 (GDF15) in human. Am. J. Surg. 220, 725–730 (2020).
Salman, A. et al. Changes in plasma growth differentiation factor-15 after laparoscopic sleeve gastrectomy in morbidly obese patients: a prospective study. J. Inflamm. Res. 14, 1365–1373 (2021).
Kleinert, M. et al. Effect of bariatric surgery on plasma GDF15 in humans. Am. J. Physiol. Endocrinol. Metab. 316, E615–E621 (2019).
Lu, J. F. et al. Camptothecin effectively treats obesity in mice through GDF15 induction. PLoS Biol. 20, e3001517 (2022).
Benichou, O. et al. Discovery, development, and clinical proof of mechanism of LY3463251, a long-acting GDF15 receptor agonist. Cell Metab. 35, 274–286.e10 (2023).
Lyu, X. et al. The antiobesity effect of GLP-1 receptor agonists alone or in combination with metformin in overweight/obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Int. J. Endocrinol. 2021, 6616693 (2021).
Cheng, L. et al. Dapagliflozin, metformin, monotherapy or both in patients with metabolic syndrome. Sci. Rep. 11, 24263 (2021).
Sen, T. et al. Association between circulating GDF-15 and cardio-renal outcomes and effect of canagliflozin: results from the CANVAS trial. J. Am. Heart Assoc. 10, e021661 (2021).
Ghidewon, M. et al. Growth differentiation factor 15 (GDF15) and semaglutide inhibit food intake and body weight through largely distinct, additive mechanisms. Diabetes Obes. Metab. 24, 1010–1020 (2022).
Anesten, F. et al. Glucagon-like peptide-1-, but not growth and differentiation factor 15-, receptor activation increases the number of interleukin-6-expressing cells in the external lateral parabrachial nucleus. Neuroendocrinology 109, 310–321 (2019).
Zhang, Y. et al. Activity-balanced GLP-1/GDF15 dual agonist reduces body weight and metabolic disorder in mice and non-human primates. Cell Metab. 35, 287–298.e4 (2023).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Garvey, W. T. et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 402, 613–626 (2023).
Parker, V. E. R. et al. Efficacy and safety of cotadutide, a dual glucagon-like peptide-1 and glucagon receptor agonist, in a randomized phase 2a study of patients with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 24, 1360–1369 (2022).
Jungnik, A. et al. Phase I studies of the safety, tolerability, pharmacokinetics and pharmacodynamics of the dual glucagon receptor/glucagon-like peptide-1 receptor agonist BI 456906. Diabetes Obes. Metab. 25, 1011–1023 (2023).
Romero-Gómez, M. et al. A phase 2a active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with nonalcoholic fatty liver disease. J. Hepatol. 79, 888–897 (2023).
Rosenstock, J. et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 402, 529–544 (2023).
Urva, S. et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: a phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet 400, 1869–1881 (2022).
Bossart, M. et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab. 34, 59–74.e10 (2022).
Acknowledgements
K.Y. is supported by the University of Toronto Department of Medicine Eliot Phillipson Clinician Scientist Training Program, Clarence Henry Trelford Clinician Scientist Award in Diabetes, and a KRESCENT Postdoctoral Fellowship. The KRESCENT program is co-sponsored by the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Institute of Health Research (CIHR). R.K. is supported by a CIHR graduate award and a Banting and Best Diabetes Centre graduate scholarship. D.Z.I.C. is supported by a Department of Medicine, University of Toronto Merit Award, and receives support from the CIHR, Diabetes Canada and the Heart and Stroke Richard Lewar Centre of Excellence. D.Z.I.C. is also the recipient of a CIHR-KFOC Teams Grant Award. T.K.T.L. is supported by CIHR grants (PJT 189957, PJT 183901, MRT-168045) and holds the Canada Research Chair (Tier 1) in Diabetes and Obesity at the Toronto General Hospital Research Institute & University of Toronto.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of content, wrote the manuscript and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
D.Z.I.C. has received consulting fees or speaking honoraria or both from Janssen, Bayer, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, Merck & Co Inc, Prometic and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, Sanofi, AstraZeneca and Merck & Co Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Alex R. Chang, Neil Docherty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yau, K., Kuah, R., Cherney, D.Z.I. et al. Obesity and the kidney: mechanistic links and therapeutic advances. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-00951-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-024-00951-7