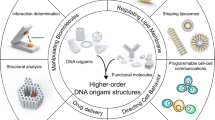
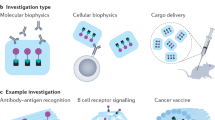
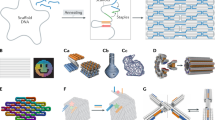
Abstract
Structural DNA nanotechnology enables the fabrication of user-defined DNA origami nanostructures (DNs) for biological applications. However, the role of DN design during cellular interactions and subsequent biodistribution remain poorly understood. Current methods for tracking DN fates in situ, including fluorescent-dye labelling, suffer from low sensitivity and dye-induced artifacts. Here we present origamiFISH, a label-free and universal method for the single-molecule fluorescence detection of DNA origami nanostructures in cells and tissues. origamiFISH targets pan-DN scaffold sequences with hybridization chain reaction probes to achieve 1,000-fold signal amplification. We identify cell-type- and DN shape-specific spatiotemporal distribution patterns within a minute of uptake and at picomolar DN concentrations, 10,000× lower than field standards. We additionally optimize compatibility with immunofluorescence and tissue clearing to visualize DN distribution within tissue cryo-/vibratome sections, slice cultures and whole-mount organoids. Together, origamiFISH enables the accurate mapping of DN distribution across subcellular and tissue barriers for guiding the development of DN-based therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the methods and findings of this study are available within the article and its Supplementary Information. The raw imaging data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Dey, S. et al. DNA origami. Nat. Rev. Methods Primers 1, 13 (2021).
Hong, F., Zhang, F., Liu, Y. & Yan, H. DNA origami: scaffolds for creating higher order structures. Chem. Rev. 117, 12584–12640 (2017).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Tseng, C. Y., Wang, W. X., Douglas, T. R. & Chou, L. Y. T. Engineering DNA nanostructures to manipulate immune receptor signaling and immune cell fates. Adv. Healthc. Mater. 11, e2101844 (2022).
Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 335, 831–834 (2012).
Li, S. et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 36, 258–264 (2018).
Zhang, Q. et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 8, 6633–6643 (2014).
Liu, S. et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 20, 421–430 (2021).
Veneziano, R. et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 15, 716–723 (2020).
Han, S., Liu, W., Yang, S. & Wang, R. Facile and label-free electrochemical biosensors for microRNA detection based on DNA origami nanostructures. ACS Omega 4, 11025–11031 (2019).
Huang, Y., Nguyen, M.-K., Natarajan, A. K., Nguyen, V. H. & Kuzyk, A. A DNA origami-based chiral plasmonic sensing device. ACS Appl. Mater. Interfaces 10, 44221–44225 (2018).
Kosuri, P., Altheimer, B. D., Dai, M., Yin, P. & Zhuang, X. Rotation tracking of genome-processing enzymes using DNA origami rotors. Nature 572, 136–140 (2019).
Kuzuya, A. et al. Nanomechanical DNA origami pH sensors. Sensors 14, 19329–19335 (2014).
Kuzuya, A., Sakai, Y., Yamazaki, T., Xu, Y. & Komiyama, M. Nanomechanical DNA origami ‘single-molecule beacons’ directly imaged by atomic force microscopy. Nat. Commun. 2, 449 (2011).
Berger, R. M. L. et al. Nanoscale FasL organization on DNA origami to decipher apoptosis signal activation in cells. Small 17, e2101678 (2021).
Dong, R. et al. DNA origami patterning of synthetic T cell receptors reveals spatial control of the sensitivity and kinetics of signal activation. Proc. Natl Acad. Sci. USA 118, e2109057118 (2021).
Hawkes, W. et al. Probing the nanoscale organisation and multivalency of cell surface receptors: DNA origami nanoarrays for cellular studies with single-molecule control. Faraday Discuss. 219, 203–219 (2019).
Hellmeier, J. et al. DNA origami demonstrate the unique stimulatory power of single pMHCs as T cell antigens. Proc. Natl Acad. Sci. USA 118, e2016857118 (2021).
Kern, N., Dong, R., Douglas, S. M., Vale, R. D. & Morrissey, M. A. Tight nanoscale clustering of Fcγ receptors using DNA origami promotes phagocytosis. eLife 10, e68311 (2021).
Bertosin, E. et al. Cryo-electron microscopy and mass analysis of oligolysine-coated DNA nanostructures. ACS Nano 15, 9391–9403 (2021).
Endo, M. & Sugiyama, H. Direct observation of dynamic movement of DNA molecules in DNA origami imaged using high-speed AFM. Methods Mol. Biol. 1814, 213–224 (2018).
Helmig, S. et al. Single molecule atomic force microscopy studies of photosensitized singlet oxygen behavior on a DNA origami template. ACS Nano 4, 7475–7480 (2010).
Kabiri, Y. et al. Visualization of unstained DNA nanostructures with advanced in-focus phase contrast TEM techniques. Sci. Rep. 9, 7218 (2019).
Silvester, E. et al. DNA origami signposts for identifying proteins on cell membranes by electron cryotomography. Cell 184, 1110–1121.e16 (2021).
Ponnuswamy, N. et al. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 8, 15654 (2017).
Bastings, M. M. C. et al. Modulation of the cellular uptake of DNA origami through control over mass and shape. Nano Lett. 18, 3557–3564 (2018).
Liang, L. et al. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew. Chem. Int. Ed. 53, 7745–7750 (2014).
Wang, P. et al. Visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells. J. Am. Chem. Soc. 140, 2478–2484 (2018).
Green, C. M., Mathur, D. & Medintz, I. L. Understanding the fate of DNA nanostructures inside the cell. J. Mater. Chem. B 8, 6170–6178 (2020).
Koga, M. M., Comberlato, A., Rodríguez-Franco, H. J. & Bastings, M. M. C. Strategic insights into engineering parameters affecting cell type-specific uptake of DNA-based nanomaterials. Biomacromolecules 23, 2586–2594 (2022).
Lacroix, A., Vengut-Climent, E., de Rochambeau, D. & Sleiman, H. F. Uptake and fate of fluorescently labeled DNA nanostructures in cellular environments: a cautionary tale. ACS Cent. Sci. 5, 882–891 (2019).
Hedegaard, S. F. et al. Fluorophore labeling of a cell-penetrating peptide significantly alters the mode and degree of biomembrane interaction. Sci. Rep. 8, 6327 (2018).
Hughes, L. D., Rawle, R. J. & Boxer, S. G. Choose your label wisely: water-soluble fluorophores often interact with lipid bilayers. PLoS ONE 9, e87649 (2014).
Zacharias, D. A., Violin, J. D., Newton, A. C. & Tsien, R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002).
Chen, A. K., Cheng, Z., Behlke, M. A. & Tsourkas, A. Assessing the sensitivity of commercially available fluorophores to the intracellular environment. Anal. Chem. 80, 7437–7444 (2008).
Kretschy, N., Sack, M. & Somoza, M. M. Sequence-dependent fluorescence of Cy3- and Cy5-labeled double-stranded DNA. Bioconjugate Chem. 27, 840–848 (2016).
Srisantitham, S., Sukwattanasinitt, M. & Unarunotai, S. Effect of pH on fluorescence quenching of organic dyes by graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 550, 123–131 (2018).
Zheng, Q., Jockusch, S., Zhou, Z. & Blanchard, S. C. The contribution of reactive oxygen species to the photobleaching of organic fluorophores. Photochem. Photobiol. 90, 448–454 (2014).
Choi, H. M. T., Beck, V. A. & Pierce, N. A. Next-generation in situ hybridization chain reaction: higher gain, lower cost, greater durability. ACS Nano 8, 4284–4294 (2014).
Choi, H. M. T. et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Seeman, N. C. & Sleiman, H. F. DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2017).
Tsuneoka, Y. & Funato, H. Modified in situ hybridization chain reaction using short hairpin DNAs. Front. Mol. Neurosci. 13, 75 (2020).
Liu, X. et al. DNA framework-encoded mineralization of calcium phosphate. Chem 6, 472–485 (2020).
Maezawa, T. et al. DNA density-dependent uptake of DNA origami-based two-or three-dimensional nanostructures by immune cells. Nanoscale 12, 14818–14824 (2020).
Zeng, Y. C. et al. Optimizing CpG spatial distribution with DNA origami for Th1-polarized therapeutic vaccination. Preprint at bioRxiv https://doi.org/10.1101/2022.06.08.495340 (2022).
Shaffer, S. M., Wu, M.-T., Levesque, M. J. & Raj, A. Turbo FISH: a method for rapid single molecule RNA FISH. PLoS ONE 8, e75120 (2013).
Shapero, K. et al. Time and space resolved uptake study of silica nanoparticles by human cells. Mol. Biosyst. 7, 371–378 (2011).
Bratu, D. P., Cha, B.-J., Mhlanga, M. M., Kramer, F. R. & Tyagi, S. Visualizing the distribution and transport of mRNAs in living cells. Proc. Natl Acad. Sci. USA 100, 13308–13313 (2003).
Tübing, F. et al. Dendritically localized transcripts are sorted into distinct ribonucleoprotein particles that display fast directional motility along dendrites of hippocampal neurons. J. Neurosci. 30, 4160–4170 (2010).
Batish, M., van den Bogaard, P., Kramer, F. R. & Tyagi, S. Neuronal mRNAs travel singly into dendrites. Proc. Natl Acad. Sci. USA 109, 4645–4650 (2012).
Mäger, I., Langel, K., Lehto, T., Eiríksdóttir, E. & Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 1818, 502–511 (2012).
Hendzel, M. J. et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997).
Zhang, Y.-N., Poon, W., Sefton, E. & Chan, W. C. W. Suppressing subcapsular sinus macrophages enhances transport of nanovaccines to lymph node follicles for robust humoral immunity. ACS Nano 14, 9478–9490 (2020).
Schnell, S. A., Staines, W. A. & Wessendorf, M. W. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 47, 719–730 (1999).
Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Wang, Y. et al. DNA origami penetration in cell spheroid tissue models is enhanced by wireframe design. Adv. Mater. 33, e2008457 (2021).
Schnitzbauer, J., Strauss, M. T., Schlichthaerle, T., Schueder, F. & Jungmann, R. Super-resolution microscopy with DNA-PAINT. Nat. Protoc. 12, 1198–1228 (2017).
Jungmann, R. et al. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat. Methods 11, 313–318 (2014).
Sasaki, H. M., Kishi, J. Y., Wu, C.-T., Beliveau, B. J. & Yin, P. Quantitative multiplexed imaging of chromatin ultrastructure with Decode-PAINT. Preprint at bioRxiv https://doi.org/10.1101/2022.08.01.502089 (2022).
Wang, Y. et al. EASI-FISH for thick tissue defines lateral hypothalamus spatio-molecular organization. Cell 184, 6361–6377.e24 (2021).
Shah, S. et al. Single-molecule RNA detection at depth by hybridization chain reaction and tissue hydrogel embedding and clearing. Development 143, 2862–2867 (2016).
Wang, W. X. & Lefebvre, J. L. Morphological pseudotime ordering and fate mapping reveal diversification of cerebellar inhibitory interneurons. Nat. Commun. 13, 3433 (2022).
Eklund, A. S., Comberlato, A., Parish, I. A., Jungmann, R. & Bastings, M. M. C. Quantification of strand accessibility in biostable DNA origami with single-staple resolution. ACS Nano 15, 17668–17677 (2021).
Li, Y. et al. Genome-wide CRISPR screen for Zika virus resistance in human neural cells. Proc. Natl Acad. Sci. USA 116, 9527–9532 (2019).
Rezende, R. M., Lopes, M. E., Menezes, G. B. & Weiner, H. L. Visualizing lymph node structure and cellular localization using ex-vivo confocal microscopy. J. Vis. Exp. e59335 (2019).
Acknowledgements
W.X.W. acknowledges the PRiME initiative for fellowship support. T.R.D. acknowledges the Barbara and Frank Milligan Graduate fellowship. Y.L. acknowledges NSERC Discovery Grant (RGPIN-2020-06345); Medicine by Design, Simons Foundation (479754); Brain Canada, Stem Cell Network (ECI-14); Can-GARD; and Brain & Behavior Research Foundation (29772). J.M. acknowledges Brain Canada (FL2021-BCF-Sickkids), Stem Cell Network (ECR-C4R1-4), NSERC Discovery Grant (RGPIN-2019-06938) and The Hospital for Sick Children. L.Y.T.C. acknowledges NSERC Discovery Grant (RGPIN-2020-05966), Medicine by Design (MbDNI-2019-01, MbDNI-2020-01) and the Canadian Foundation for Innovation (39262) for funding. We thank W. Shih for sharing the DN barrel design sequences. We thank members of the Chou lab for helpful comments on this paper. We thank P. Paroutis and the Hospital for Sick Children imaging facility for instrument support and guidance on light-sheet microscopy. We thank J. Lefebvre for the generous gift of antibodies, reagents and use of equipment.
Author information
Authors and Affiliations
Contributions
W.X.W. and L.Y.T.C. conceptualized the project and designed the experiments. W.X.W. performed the experiments with assistance from T.R.D., H.Z., A.B., M.R. and W.T. W.X.W. performed the analyses. Z.J., J.M., Y.L., Y.S. and L.Y.T.C. participated in the data interpretation and provided supervision. W.X.W. and L.Y.T.C. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Amelie Heuer-Jungemann, Tania Patiño and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Notes 1–4 and Methods.
Supplementary Data 1
Supplementary Tables 1 and 2.
Supplementary Data 2
Raw data for Supplementary Figs. 4 and 8–10.
Supplementary Video 1
DN microinjection video.
Source data
Source Data Fig. 2
Raw data for the graphs in Fig. 2.
Source Data Fig. 3
Raw data for the graphs in Fig. 3.
Source Data Fig. 4
Raw data for the graphs in Fig. 4.
Source Data Fig. 5
Raw data for the graphs in Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W.X., Douglas, T.R., Zhang, H. et al. Universal, label-free, single-molecule visualization of DNA origami nanodevices across biological samples using origamiFISH. Nat. Nanotechnol. 19, 58–69 (2024). https://doi.org/10.1038/s41565-023-01449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01449-5
This article is cited by
-
Imaging DNA origami by fluorescence in situ hybridization
Nature Nanotechnology (2024)
-
Biosorption of lead ion by lactic acid bacteria and the application in wastewater
Archives of Microbiology (2024)