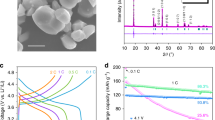
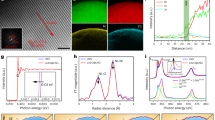
Abstract
Coatings and surface passivation are sought to protect high-energy-density cathodes in lithium-ion batteries, which suffer from labile oxygen loss and fast degradations. Here we develop the theory underlying the high-voltage-induced oxygen evolution crisis and report a lanthurizing process to regulate the near-surface structure of energy materials beyond conventional surface doping. Using LiCoO2 as an example and generalizing to Co-lean/free high-energy-density layered cathodes, we demonstrate effective surface passivation, suppressed surface degradation and improved electrochemical performance. High-voltage cycling stability has been greatly enhanced, up to 4.8 V versus Li+/Li, including in practical pouch-type full cells. The superior performance is rooted in the engineered surface architecture and the reliability of the synthesis method. The designed surface phase stalls oxygen evolution reaction at high voltages. It illustrates processing opportunities for surface engineering and coating by high-oxygen-activity passivation, selective chemical alloying and strain engineering using wet chemistry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings in the present work are available in the manuscript and supplementary information.
References
Yang, Q. et al. Surface-protected LiCoO2 with ultrathin solid oxide electrolyte film for high-voltage lithium ion batteries and lithium polymer batteries. J. Power Sources 388, 65–70 (2018).
Huggins, R. A. Do you really want an unsafe battery? J. Electrochem. Soc. 160, A3001 (2013).
Sun, C. et al. High-voltage cycling induced thermal vulnerability in LiCoO2 cathode: cation loss and oxygen release driven by oxygen vacancy migration. ACS Nano 14, 6181–6190 (2020).
Hu, E. et al. Oxygen-redox reactions in LiCoO2 cathode without O–O bonding during charge–discharge. Joule 5, 720–736 (2021).
Ong, S. P., Wang, L., Kang, B. & Ceder, G. Li–Fe–P–O2 phase diagram from first principles calculations. Chem. Mater. 20, 1798–1807 (2008).
Ong, S. P., Jain, A., Hautier, G., Kang, B. & Ceder, G. Thermal stabilities of delithiated olivine MPO4 (M = Fe, Mn) cathodes investigated using first principles calculations. Electrochem. Commun. 12, 427–430 (2010).
Liu, M. et al. Spinel compounds as multivalent battery cathodes: a systematic evaluation based on ab initio calculations. Energy Environ. Sci. 8, 964–974 (2015).
Li, J. et al. Structural origin of the high-voltage instability of lithium cobalt oxide. Nat. Nanotechnol. 16, 599–605 (2021).
Zhu, Z. et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment. Nat. Energy 4, 1049–1058 (2019).
Zhang, S., Ma, J., Hu, Z., Cui, G. & Chen, L. Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials. Chem. Mater. 31, 6033–6065 (2019).
Zhang, F. et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 11, 3050 (2020).
Dong, Y-H. & Li, J. Oxide cathodes: functions, instabilities, self healing, and degradation mitigations. Chem. Rev. (in the press).
Heard, D. M. & Lennox, A. J. Electrode materials in modern organic electrochemistry. Angew. Chem. Int. Ed. 59, 18866–18884 (2020).
Zhao, E. et al. Structural and mechanistic revelations on high capacity cation-disordered Li-rich oxides for rechargeable Li-ion batteries. Energy Storage Mater. 16, 354–363 (2019).
Liu, H. et al. Insight into the role of metal–oxygen bond and O 2p hole in high-voltage cathode LiNixMn2–xO4. J. Phys. Chem. C. 121, 16079––16087 (2017).
Antipin, D. & Risch, M. Trends of epitaxial perovskite oxide films catalyzing the oxygen evolution reaction in alkaline media. J. Phys. Energy 2, 032003 (2020).
Zhu, Z. et al. Gradient-morph LiCoO2 single crystals with stabilized energy density above 3,400 W h L−1. Energy Environ. Sci. 13, 1865–1878 (2020).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Manthiram, A. & Goodenough, J. B. Lithium-based polyanion oxide cathodes. Nat. Energy 6, 844–845 (2021).
Thackeray, M. & Amine, K. LiMn2O4 spinel and substituted cathodes. Nat. Energy 6, 566–566 (2021).
Huang, Y. et al. Lithium manganese spinel cathodes for lithium‐ion batteries. Adv. Energy Mater. 11, 2000997 (2021).
Yoon, M. et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 6, 362–371 (2021).
Parrish, G. Carburizing: Microstructures and Properties (Asm International, 1999).
Zhou, M. et al. Enhancing the intrinsic activity and stability of perovskite cobaltite at elevated temperature through surface stress. Small 17, 2104144 (2021).
Orlovskaya, N., Kleveland, K., Grande, T. & Einarsrud, M. A. Mechanical properties of LaCoO3 based ceramics. J. Eur. Ceram. Soc. 20, 51–56 (2000).
Wang, J. et al. Ultrathin LiCoO2 nanosheets: an efficient water-oxidation catalyst. ACS Appl. Mater. Inter. 9, 7100–7107 (2017).
Zhao, R. et al. The origin of heavy element doping to relieve the lattice thermal vibration of layered materials for high energy density Li ion cathodes. J. Mater. Chem. A 8, 12424–12435 (2020).
Seo, D. H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Yoon, M. et al. Unveiling nickel chemistry in stabilizing high‐voltage cobalt‐rich cathodes for lithium‐ion batteries. Adv. Funct. Mater. 30, 1907903 (2020).
Dong, Y. et al. Potential jumps at transport bottlenecks cause instability of nominally ionic solid electrolytes in electrochemical cells. Acta Mater. 199, 264–277 (2020).
Inaguma, Y. et al. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 86, 689–693 (1993).
Kang, K., Meng, Y., Breger, J., Grey, C. P. & Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311, 977–980 (2006).
Cao, X. et al. Stabilizing anionic redox chemistry in a Mn‐based layered oxide cathode constructed by Li‐deficient pristine state. Adv. Mater. 33, 2004280 (2021).
Li, J. EML webinar overview: elastic strain engineering for unprecedented properties. Extreme Mech. Lett. 54, 101430 (2022).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Lin, F. et al. Synchrotron X-ray analytical techniques for studying materials electrochemistry in rechargeable batteries. Chem. Rev. 117, 13123–13186 (2017).
Ren, G. et al. Photon-in/photon-out endstation for studies of energy materials at beamline 02B02 of Shanghai Synchrotron Radiation Facility. Chin. Phys. B 29, 016101 (2020).
Yoon, W. S. et al. Oxygen contribution on Li-ion intercalation−deintercalation in LiCoO2 investigated by O K-edge and Co L-edge X-ray absorption spectroscopy. J. Phys. Chem. B 106, 2526–2532 (2002).
Zhao, J. et al. Electronic structure evolutions driven by oxygen vacancy in SrCoO3−x films. Sci. China Mater. 62, 1162–1168 (2019).
Acknowledgements
F.H. acknowledges support by the National Natural Science Foundation of China (grants 21871008, 11227902), the Science and Technology Commission of Shanghai (grant 18YF1427200) and the Key Research Program of Frontier Science, Chinese Academy of Sciences (grant number QYZDJ-SSW-JSC013). J.L. acknowledges support from Samsung Advanced Institute of Technology. We thank beamline 02B02 of the Shanghai Synchrotron Radiation Facility for providing the beamtime.
Author information
Authors and Affiliations
Contributions
F.H. conceived the project. J.L. developed the theory. M.C. and F.H. synthesized the materials and conducted the electrochemical measurements. Y.D. and H.X. conducted the simulations. M.C. and M.X. assembled and tested pouch-type full cells. P.D. conducted in situ DEMS measurements. H.Z. conducted sXAS measurements. S.Z. conducted XPS depth profile measurements. M.C., Y.D. and F.H. analysed the data. M.C., Y.D., J.L. and F.H. wrote the paper. All authors discussed and contributed to the writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Post-cycling morphological, structural and chemical characteristics of oxide surface and CEIs.
a, b, HRTEM near the surface of cycled P-LCO (a) and La-LCO (b). Circled in (a) are Moire effects produced by lattice distortion. c, d, Depth-resolved EELS profiles near the surface of cycled P-LCO (c) and La-LCO (d). e, f, Calculated Co L3/L2 area ratio (e) and O/Co ratio (f) of cycled P-LCO and La-LCO from (c, d). g, h, HRTEM near the surfaces of cycled P-LCO (g) and La-LCO (h), showing the CEI layer enclosed by dash lines. Scale bars: 5 nm (a, b, g, h). i–l, XPS of C 1s (i, k) and F 1s (j, l) for cycled P-LCO (i, j) and La-LCO (k, l). Details for XPS analysis are listed in Supplementary Table 5 and 6. Characterizations in (a–l) were conducted on samples after 100 cycles at 1 C and 3.0–4.6 V (vs. Li+/Li) in half cells. m, Dissolved Co in the electrolytes after 100 and 200 cycles under 1 C within 3.0–4.6 V (vs. Li+/Li) in half cells. n, o, XPS of Co 2p at the surface of the cycled graphite anodes in pouch cells using La-LCO (n) and P-LCO (o) cathodes after 200 cycles under 200 mA at full-cell voltages 3.0–4.5 V.
Extended Data Fig. 2 Generalization of lanthurizing process to Co-lean NCM and Co-free LRNM cathodes.
a, Cycling performance of P-NCM and La-NCM in coin-type half cells at 1 C at 2.8–4.4 V vs. Li+/Li. b, c, Charge–discharge profiles of P-NCM (b) and La-NCM (c) at the 5th, 50th, 100th and 150th cycles. d, Cycling performance of P-LRNM and La-LRNM in coin-type half cells at 1 C at 2.0–4.8 V vs. Li+/Li. e, f, Charge–discharge profiles of P-LRNM (e) and La-LRNM (f) at the 5th, 50th, 100th and 150th cycles.
Supplementary information
Supplementary Information
Supplementary Notes 1–3, Figs. 1–24, Tables 1–9 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, M., Dong, Y., Xie, M. et al. Stalling oxygen evolution in high-voltage cathodes by lanthurization. Nat Energy 8, 159–168 (2023). https://doi.org/10.1038/s41560-022-01179-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01179-3
This article is cited by
-
Enhanced capacity of LiCoO2 and graphite battery by using methylene methanedisulfonate as electrolyte additive
Journal of Applied Electrochemistry (2024)
-
Mitigating Lattice Distortion of High-Voltage LiCoO2 via Core-Shell Structure Induced by Cationic Heterogeneous Co-Doping for Lithium-Ion Batteries
Nano-Micro Letters (2024)
-
Sodium Formate as a Highly Efficient Sodium Compensation Additive for Sodium-Ion Batteries with a P2-Type Layered Oxide Cathode
Journal of Electronic Materials (2024)
-
Suppressing strain propagation in ultrahigh-Ni cathodes during fast charging via epitaxial entropy-assisted coating
Nature Energy (2024)
-
A Li-rich layered oxide cathode with negligible voltage decay
Nature Energy (2023)