Abstract
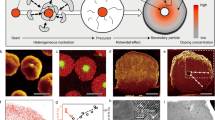
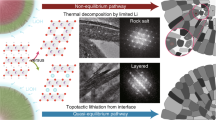
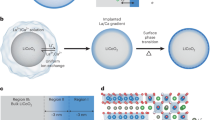
Surface reconstruction and the associated severe strain propagation have long been reported as the major cause of cathode failure during fast charging and long-term cycling. Despite tremendous attempts, no known strategies can simultaneously address the electro-chemomechanical instability without sacrificing energy and power density. Here we report an epitaxial entropy-assisted coating strategy for ultrahigh-Ni LiNixCoyMn1−x−yO2 (x ≥ 0.9) cathodes via an oriented attachment-driven reaction between Wadsley–Roth phase-based oxides and the layered-oxide cathodes. The high anti-cracking and anti-corrosion tolerances as well as the fast ionic transport of the entropy-assisted surface effectively improved the fast charging/discharging capability, wide temperature tolerance and thermal stability of the ultrahigh-Ni cathodes. Comprehensive analysis from the primary and secondary particle level to the electrode level using multi-scale in situ synchrotron X-ray probes reveals greatly reduced lattice dislocations, anisotropic lattice strain and oxygen release as well as improved bulk/local structural stability, even when charging beyond the threshold state of charge (75%) of layered cathodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are included within the article and its Supplementary Information files. Source data are provided with this paper.
Change history
07 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41560-024-01493-y
References
Li, W., Erickson, E. M. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26–34 (2020).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Xu, C. et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 20, 84–92 (2021).
Lin, F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014).
Cai, M. et al. Stalling oxygen evolution in high-voltage cathodes by lanthurization. Nat. Energy 8, 159–168 (2023).
Lin, F., Zhao, K. & Liu, Y. Heterogeneous reaction activities and statistical characteristics of particle cracking in battery electrodes. ACS Energy Lett. 6, 4065–4070 (2021).
Li, J. et al. Dynamics of particle network in composite battery cathodes. Science 376, 517–521 (2022).
Li, W., Asl, H. Y., Xie, Q. & Manthiram, A. Collapse of LiNi1–x–yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 141, 5097–5101 (2019).
Yan, P. et al. Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode. Nat. Commun. 9, 2437 (2018).
Yan, P. et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 8, 14101 (2017).
Zhan, C., Wu, T., Lu, J. & Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—a critical review. Energy Environ. Sci. 11, 243–257 (2018).
Xiang, Y. et al. Gas induced formation of inactive Li in rechargeable lithium metal batteries. Nat. Commun. 14, 177 (2023).
Xia, S. et al. Chemomechanical interplay of layered cathode materials undergoing fast charging in lithium batteries. Nano Energy 53, 753–762 (2018).
Hu, E., Wang, X., Yu, X. & Yang, X.-Q. Probing the complexities of structural changes in layered oxide cathode materials for Li-ion batteries during fast charge–discharge cycling and heating. Acc. Chem. Res. 51, 290–298 (2018).
Tanim, T. R. et al. Extended cycle life implications of fast charging for lithium-ion battery cathode. Energy Storage Mater. 41, 656–666 (2021).
Li, J. et al. Multiphase, multiscale chemomechanics at extreme low temperatures: battery electrodes for operation in a wide temperature range. Adv. Energy Mater. 11, 2102122 (2021).
Chen, Z., Qin, Y., Amine, K. & Sun, Y. K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 20, 7606–7612 (2010).
Gao, H. et al. Modifying the surface of a high-voltage lithium-ion cathode. ACS Appl. Energy Mater. 1, 2254–2260 (2018).
Yan, P. et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 3, 600–605 (2018).
Xu, G.-L. et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 4, 484–494 (2019).
Nisar, U., Muralidharan, N., Essehli, R., Amin, R. & Belharouak, I. Valuation of surface coatings in high-energy density lithium-ion battery cathode materials. Energy Storage Mater. 38, 309–328 (2021).
Ren, J. et al. Strong yet ductile nanolamellar high-entropy alloys by additive manufacturing. Nature 608, 62–68 (2022).
Zeng, Y. et al. High-entropy mechanism to boost ionic conductivity. Science 378, 1320–1324 (2022).
Yang, B. et al. High-entropy enhanced capacitive energy storage. Nat. Mater. 21, 1074–1080 (2022).
Ma, Y. et al. High-entropy energy materials: challenges and new opportunities. Energy Environ. Sci. 14, 2883–2905 (2021).
Zhang, R. et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 610, 67–73 (2022).
Tan, X. et al. High-entropy surface complex stabilized LiCoO2 cathode. Adv. Energy Mater. https://doi.org/10.1002/aenm.202300147 (2023).
Zhao, C., Ding, F., Lu, Y., Chen, L. & Hu, Y.-S. High-entropy layered oxide cathodes for sodium-ion batteries. Angew. Chem. Int. Ed. 59, 264–269 (2020).
Fu, F. et al. Entropy and crystal-facet modulation of P2-type layered cathodes for long-lasting sodium-based batteries. Nat. Commun. 13, 282 (2022).
Penn, R. L. & Banfield, J. F. Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science 281, 969–971 (1998).
Liu, Y. et al. Oriented attachment revisited: does a chemical reaction occur? Matter 1, 690–704 (2019).
Griffith, K. J., Wiaderek, K. M., Cibin, G., Marbella, L. E. & Grey, C. P. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature 559, 556–563 (2018).
MacArthur, K. E. et al. Optimizing experimental conditions for accurate quantitative energy-dispersive x-ray analysis of interfaces at the atomic scale. Microsc. Microanal. 27, 528–542 (2021).
Lu, P., Moya, J. M., Yuan, R. & Zuo, J. M. Studies of X-ray localization and thickness dependence in atomic-scale elemental mapping by STEM energy-dispersive X-ray spectroscopy using single-frame scanning method. Ultramicroscopy 186, 23–29 (2018).
Yuan, K. et al. Self-ball milling strategy to construct high-entropy oxide coated LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance. J. Adv. Ceram. 11, 882–892 (2022).
Mao, Y. et al. High-voltage charging-induced strain, heterogeneity, and microcracks in secondary particles of a nickel-rich layered cathode material. Adv. Funct. Mater. 29, 1900247 (2019).
Liu, T. et al. Rational design of mechanically robust Ni-rich cathode materials via concentration gradient strategy. Nat. Commun. 12, 6024 (2021).
Xu, C., Reeves, P. J., Jacquet, Q. & Grey, C. P. Phase behavior during electrochemical cycling of Ni‐rich cathode materials for Li‐ion batteries. Adv. Energy Mater. 11, 2003404 (2021).
Wang, L. G., Liu, T. C., Wu, T. P. & Lu, J. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 611, 61–67 (2022).
Li, L. X., Xie, Y. Y., Maxey, E. & Harder, R. Methods for operando coherent X-ray diffraction of battery materials at the advanced photon source. J. Synchrotron Radiat. 26, 220–229 (2019).
Ulvestad, A. et al. Topological defect dynamics in operando battery nanoparticles. Science 348, 1344–1347 (2015).
Singer, A. et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging. Nat. Energy 3, 641–647 (2018).
Liu, T. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305–312 (2022).
Estandarte, A. K. C. et al. Operando bragg coherent diffraction imaging of LiNi0.8Mn0.1Co0.1O2 primary particles within commercially printed NMC811 electrode sheets. ACS Nano 15, 1321–1330 (2021).
Tallman, K. R. et al. Nickel-rich nickel manganese cobalt (NMC622) cathode lithiation mechanism and extended cycling effects using operando X-ray absorption spectroscopy. J. Phys. Chem. C 125, 58–73 (2021).
Tsai, Y. W., Lee, J. F., Liu, D. G. & Hwang, B. J. In situ X-ray absorption spectroscopy investigations of a layered LiNi0.65Co0.25Mn0.1O2 cathode material for rechargeable lithium batteries. J. Mater. Chem. 14, 958–965 (2004).
Ma, L., Wang, L., Liu, T., Wu, T. & Lu, J. Probing distinctive redox mechanism in Ni-rich cathode via real-time quick X-ray absorption spectroscopy. Small Methods 7, 2201173 (2023).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020).
Asl, H. Y. & Manthiram, A. Reining in dissolved transition metal ions. Science 369, 140–141 (2020).
Rehr, J. J. & Albers, R. C. Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 72, 621–654 (2000).
Yoon, W.-S. et al. Investigation of the charge compensation mechanism on the electrochemically Li-ion deintercalated Li1-xCo1/3Ni1/3Mn1/3O2 electrode system by combination of soft and hard X-ray absorption spectroscopy. J. Am. Chem. Soc. 127, 17479–17487 (2005).
Yoon, W.-S., Grey, C. P., Balasubramanian, M., Yang, X.-Q. & McBreen, J. In situ X-ray absorption spectroscopic study on LiNi0.5Mn0.5O2 cathode material during electrochemical cycling. Chem. Mater. 15, 3161–3169 (2003).
Liu, X. et al. Origin and regulation of oxygen redox instability in high-voltage battery cathodes. Nat. Energy 7, 808–817 (2022).
Deng, J. et al. The Velociprobe: an ultrafast hard X-ray nanoprobe for high-resolution ptychographic imaging. Rev. Sci. Instrum. 90, 083701 (2019).
Acknowledgements
Research at the Argonne National Laboratory was funded by the US Department of Energy, Vehicle Technologies Office. Use of the APS and the Center for Nanoscale Materials, both Office of Science user facilities, was supported by the US Department of Energy, Office of Science and Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. G.-L.X. and K.A. thank the Clean Vehicle Consortium, US–China Clean Energy Research Center (CERC-CVC2), for support.
Author information
Authors and Affiliations
Contributions
G.-L.X., C.Z. and K.A. conceived the ideas. G.-L.X. and K.A. supervised the project. C.W., C.Z., J.-T. L. and S.-G.S. developed and synthesized cathode materials. C.Z. conducted electrochemical tests for various cells. Y.L., C.Z., G.W. and X.Z. conducted the TEM and SEM characterizations. Z.Y. conducted the cross-section SEM characterizations. C.Z., X.L., T.L. and W.X. carried out synchrotron HEXRD studies of various materials. L.L. and C.Z. conducted the in situ CMCD and BCDI characterization and analysis with the help of J. Diao, L.W., W.C., R.H. and I.R. I.H., C.S. and C.Z. conducted the XAS studies of various materials. J. Deng conducted the X-ray ptychography and data processing with the help of Y.J. and T.B. Y.Q. and W.L. conducted DSC tests. G.-L.X. and C.Z. drafted the paper with the help of all the other authors.
Corresponding authors
Ethics declarations
Competing interests
For G.-L.X., C.Z., Y.L. and K.A., a US non-provisional patent application, patent application no. 17/955,092, has been filed for this work. The patent is related to the coating strategy reported in this work and submitted by Argonne National Laboratory. All other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Ben Breitung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, Tables 1–6 and discussion.
Supplementary Video 1
Complete evolution patterns of in situ coherent multi-crystal diffraction characterization.
Source data
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, C., Wang, C., Liu, X. et al. Suppressing strain propagation in ultrahigh-Ni cathodes during fast charging via epitaxial entropy-assisted coating. Nat Energy 9, 345–356 (2024). https://doi.org/10.1038/s41560-024-01465-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-024-01465-2
This article is cited by
-
Entropy-assisted epitaxial coating
Nature Energy (2024)