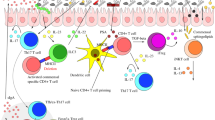
Abstract
The gut microbiota play a pivotal role in human health. Emerging evidence indicates that gut microbes participate in the progression of tumorigenesis through the generation of carcinogenic metabolites. However, the underlying molecular mechanism is largely unknown. In the present study we show that a tryptophan metabolite derived from Peptostreptococcus anaerobius, trans-3-indoleacrylic acid (IDA), facilitates colorectal carcinogenesis. Mechanistically, IDA acts as an endogenous ligand of an aryl hydrocarbon receptor (AHR) to transcriptionally upregulate the expression of ALDH1A3 (aldehyde dehydrogenase 1 family member A3), which utilizes retinal as a substrate to generate NADH, essential for ferroptosis-suppressor protein 1(FSP1)-mediated synthesis of reduced coenzyme Q10. Loss of AHR or ALDH1A3 largely abrogates IDA-promoted tumour development both in vitro and in vivo. It is interesting that P. anaerobius is significantly enriched in patients with colorectal cancer (CRC). IDA treatment or implantation of P. anaerobius promotes CRC progression in both xenograft model and ApcMin/+ mice. Together, our findings demonstrate that targeting the IDA–AHR–ALDH1A3 axis should be promising for ferroptosis-related CRC treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The survival curve data of AHR gene expression data in human cancer tissues were derived from The Cancer Genome Atlas (TCGA) database and in human cancer cell lines from the Cancer Cell Line Encyclopedia (CCLE; https://portals.broadinstitute.org/ccle). The correlation of AHR and ALDH1A3 in human cancer tissues was derived from Gene Expression Profiling Interactive Analysis (http://gepia2.cancer-pku.cn). The expression of AHR and ALDH1A3 was derived from Oncomine (https://www.oncomine.org). ChIP–seq data of AHR are available from the database (http://chip-atlas.org). RNA-seq data are provided as Supplementary tables. Source data are provided with this paper.
Code availability
All packages used for data analysis are publicly available. No customized code was generated for the present study. All scripts used for bulk RNA-seq data analyses in the present study are provided as Supplementary tables.
References
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Blander, J. M., Longman, R. S., Iliev, I. D., Sonnenberg, G. F. & Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 18, 851–860 (2017).
Roy, S. & Trinchieri, G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer 17, 271–285 (2017).
Tilg, H., Zmora, N., Adolph, T. E. & Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 20, 40–54 (2020).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 33, 988–1000.e1007 (2021).
Long, X. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 4, 2319–2330 (2019).
Louis, P., Hold, G. L. & Flint, H. J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672 (2014).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Soula, M. et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16, 1351–1360 (2020).
Chu, B. et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 21, 579–591 (2019).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Wang, W. et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Zhang, Y. et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20, 1181–1192 (2018).
Cui, W., Liu, D., Gu, W. & Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 28, 2536–2551 (2021).
Fiore, A. et al. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol. Cell 82, 920–932.e927 (2022).
Heath-Pagliuso, S. et al. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 37, 11508–11515 (1998).
Wlodarska, M. et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe 22, 25–37.e26 (2017).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Jin, D. Y. et al. A genome-wide CRISPR–Cas9 knockout screen identifies FSP1 as the warfarin-resistant vitamin K reductase. Nat. Commun. 14, 828 (2023).
Duan, J. J., Cai, J., Guo, Y. F., Bian, X. W. & Yu, S. C. ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int. J. Cancer 139, 965–975 (2016).
Dodd, D. et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652 (2017).
Yachida, S. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976 (2019).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 168–174 (2023).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 (2010).
Ehrlich, A. K. et al. AhR activation increases IL-2 production by alloreactive CD4+ T cells initiating the differentiation of mucosal-homing Tim3+ Lag3+ Tr1 cells. Eur. J. Immunol. 47, 1989–2001 (2017).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Schulz, V. J. et al. Non-dioxin-like AhR ligands in a mouse peanut allergy model. Toxicol. Sci. 128, 92–102 (2012).
Singh, N. P. et al. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J. Immunol. 196, 1108–1122 (2016).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Mishima, E. et al. Drugs repurposed as antiferroptosis agents suppress organ damage, Including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 31, 280–296 (2020).
Zeitler, L. et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. eLife 10, e64806 (2021).
Kawajiri, K. et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl Acad. Sci. USA 106, 13481–13486 (2009).
Lee, R. et al. Synthetic essentiality of tryptophan 2,3-dioxygenase 2 in APC-mutated colorectal cancer. Cancer Discov. 12, 1702–1717 (2022).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e1826 (2023).
Zhang, Q. et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8+ T cell immunity. Cell Metab. 35, 943–960.e949 (2023).
Liu, D. et al. Tryptophan metabolism acts as a new anti-ferroptotic pathway to mediate tumor growth. Adv. Sci. 10, e2204006 (2023).
Cattaneo, C. M. et al. Tumor organoid-T-cell coculture systems. Nat. Protoc. 15, 15–39 (2020).
Ping, Y. Q. et al. Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature 589, 620–626 (2021).
Collignon, E. et al. m6A RNA methylation orchestrates transcriptional dormancy during paused pluripotency. Nat. Cell Biol. 25, 1279–1289 (2023).
Duplaquet, L. et al. KDM6A epigenetically regulates subtype plasticity in small cell lung cancer. Nat. Cell Biol. 25, 1346–1358 (2023).
Hoetker, M. S. et al. H3K36 methylation maintains cell identity by regulating opposing lineage programmes. Nat. Cell Biol. 25, 1121–1134 (2023).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant nos. 2022YFA0912600 to B.C. and 2021YFA1101300 and 2020YFA0112503 to R.C.), National Natural Science Foundation of China (grant nos. 32000515 to B.C., 82325025 to S.Z. and 82030029 and 81970882 to R.C.), Natural Science Foundation of Shandong Province (grant no. ZR2020QC074 to B.C.), Taishan Scholars Program (grant no. TSQN201909030 to B.C.) of Shandong Province, Cutting Edge Development Fund of Advanced Medical Research Institute (to B.C.), Strategic Priority Research Program of the Chinese Academy of Science (grant no. XDA16010303 to R.C.), Natural Science Foundation from Jiangsu Province (grant no. BE2019711 to R.C.) and Science and Technology Department of Sichuan Province (grant no. 2021YFS0371 to R.C.). Model diagrams of the animal experiment in Fig. 7 were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
W.C., M.G. and D.L. performed most experiments with assistance from P.X., C.Y., C.L., Y.Y., Yudan Z., J.L. and X.F. Yongchun Z. and H.H. performed organoid experiments. L.D., S.S. and Y.X. collected clinical samples. J.C. and Z.H. analysed intestinal microbial data. B.C. and S.Z. designed the experiments. B.C. supervised the study, established collaborations, allocated funding for the present study and wrote most of the manuscript with the assistance of J.S., R.C., W.G. and S.Z. B.C. had the role of conceptualization, design and preparation of the manuscript and R.C. and S.Z. in preparation of the manuscript. All the authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Ömer Yilmaz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 IDA but not any other tryptophan metabolites renders cells resistant to ferroptosis.
(a, b) Dose-dependent toxicity of erastin induced cell death of HT1080 (a) and 786-O cells (b) supplemented with IDA (50 μM). Cell viability was assessed 24 h thereafter using CCK8. (c) Chemical structure of IDA and IPA. (d) Cell viability of HT1080 and 786-O cells treated with RSL3 (1 μM) and indicated concentration of IDA or IPA for 24 h. (e, f) Representative phase-contrast images of HT1080 cells (e) incubated with IDA (50 μM) for 12 h were treated with RSL3 (200 nM) and Fer-1 (1 μM). Dead cells stained with Sytox Green were calculated (f). Scale bars, 50 µm. (g) Cell death of HT1080 cells treated with erastin (5 μM), IDA (50 μM) and Fer-1 (1 μM) for 24 h. (h) FACS sequential gating strategies for C11-BODIPY 581/591 staining of lipid peroxidation were shown. (i) Chemical structure of tryptophan derivates derived from gut microbiota. (j-p) Cell death in HT1080 cells treated with RSL3 (200 nM) and indicated concentration of tryptophan derivates ranging from 10 μM to 500 μM for 16 h. Data and error bars are mean ± s.d., n = 3 biological independent experiments in a, b, d, f, g and j-p. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 2 IDA-mediated ferroptosis inhibition is independent of RTA activity and promotes tumour growth both in vitro and in vivo.
(a, b) Cell viability in HT1080 (a) and HT29 cells (b) treated with erastin and the indicated tryptophan metabolites for 24 h. Tryptophan (50 μM), IDA (50 μM), IPA (50 μM), 3-indole (50 μM), Indole-3-acetic acid (IAA, 50 μM), indole-3-aldehyde (IAld, 50 μM) and Indole-3-lactic acid (ILA, 50 μM). (c) Cell death of MC38 cells treated with RSL3 (5 μM), IDA (50 μM) and Fer-1 (1 μM) for 12 h. (d) Predicted lipophilicity of the tryptophan metabolites (https://molinspiration.com/cgi-bin/properties). (e) DPPH assay to test the ability of the tryptophan metabolites as radical trapping agent. (f) HT29 3D Spheroids treated with RSL3 (5 μM), IDA (100 μM) and Lipro-1(1 μM) for 48 h and then sytox green positive area was calculated. (g) MC38 3D Spheroids treated with RSL3 (5 μM), IDA (100 μM) and Lipro-1(1 μM) for 48 h and then sytox green positive area was calculated. (h) HT29 organoids treated with RSL3 (5 μM), IDA (200 μM) and Lipro-1(1 μM) for 72 h and then sytox green positive area was calculated. (i) LC-MS analysis of IDA content in plasma of C57BL/6 J mice via intraperitoneal administration of 50 mg/kg IDA. Data and error bars are mean ± s.d., n = 3 biological independent experiments in a-c, e-h. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 3 IDA-mediated ferroptosis resistance is dependent of AHR.
(a) Chemical structure of AHR antagonists BAY-218 and SR1. (b) Cell viability of HT1080 cells treated with RSL3, IDA (50 μM), BAY-218 (10 μM) and SR1 (10 μM) for 24 h. (c) Western blot analysis of HT1080 cells expressing shRNA-ctrl or AHR-shRNA. (d) Cell viability in HT1080 cells expressing shRNA-ctrl or AHR-shRNA treated with RSL3 (200 nM) and IDA (50 μM) for 24 h. (e) Western blot analysis of HT1080 cells expressing sg-ctrl or sg-AHR. (f, g) Cell viability (f) and lipid peroxidation (g) of HT1080 cells expressing sg-ctrl or AHR-sg treated with RSL3 (200 nM) and IDA (50 μM). (h) Relative mRNA levels of AHR in MC38 cells transfected with AHR siRNA. (I, j) Cell viability (i) and lipid peroxidation (j) of MC38 cells with AHR siRNA treated with RSL3 (5 μM) and IDA (50 μM). (k) Western blot analysis of AHR in HT29 WT and AHR KO cells. (l) Western blot analysis of AHR in HT1080 WT and AHR KO cells. (m) Cell growth curve of HT29 WT and AHR KO cells for the indicated time. (n) Cell growth curve of HT1080 WT and AHR KO cells for the indicated time. (o, p) Cell death of HT29 AHR KO (o) and HT1080 AHR KO cells (p) treated with RSL3 and IDA (50 μM) for 8 h. (q) Western blot analysis of AHR in HT1080 AHR KO cells with ectopic expression of AHR. (r) HT1080 AHR KO cells with ectopic expression of AHR treated with RSL3 (200 nM) and IDA (50 μM) and then sytox green positive area was calculated. (s) Lipid peroxidation of HT1080 AHR KO cells with ectopic expression of AHR treated with RSL3 (200 nM) and IDA (50 μM). (t) Cell viability of WT, AHR KO, NRF2 KO HT1080 cells upon RSL3 and a titration of IDA treatment. The concentration of IDA is 5, 10, 20, 50, 100, 200 μM. The Western blot experiments were repeated three times independently with similar results in c, e, k, l and q. Data and error bars are mean ± s.d., n = 3 biological independent experiments in b, d, f-j, m-p and r-t. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 4 The ligand-binding domain of AHR is critical for IDA-mediated ferroptosis.
(a) Schematic representation of the FlAsH-BRET assay design. The Nluc was inserted at the N terminus of wild-type AHR, and the FlAsH motif were inserted in the designated positions in the figure. FlAsH motifs are labelled in red. The specific residues that interact with the ligand are highlighted in yellow. (b, c) Representative dose response curve of the BCM-induced BRET ratio in HEK293 cells overexpressing two FlAsH-BRET sensors 309 (b) and 301(c) sites using FlAsH-BRET assay. (d) Western blotting showing AHR protein levels in nucleus and cytoplasm fractions from HT29 cells upon IDA (50 μM) treatment for 2 h. (e, f) mRNA levels of AHR target genes in HT1080 (e) and HT29 cells (f) upon supplementation of tryptophan metabolites. Tryptophan (50 μM), IDA (50 μM), IPA (50 μM), 3-indole (50 μM), Indole-3-acetic acid (IAA, 50 μM), indole-3-aldehyde (IAld, 50 μM) and Indole-3-lactic acid (ILA, 50 μM). (g) Schematic diagram of WT, ligand binding domain-deficient (ΔLBD) and transactivation domain-deficient (ΔTAD) AHR. (h) Western blot analysis of expression of WT, ΔLBD and ΔTAD AHR in HT1080 AHR KO cells. (i) Cell death in HT1080 AHR KO cells expressing WT, ΔLBD and ΔTAD AHR treated with RSL3 (200 nM) and IDA (50 μM) for 8 h. (j) Western blot analysis of HT1080 AHR KO cells transfected with indicated mutant AHR constructs. (k) mRNA levels of CYP1A2 in HT1080 AHR KO cells transfected with indicated mutant AHR constructs upon IDA treatment. (l) Cell death of HT1080 AHR KO cells transfected with indicated mutant AHR constructs. The Western blot experiments were repeated three times independently with similar results in d, h and j. Data and error bars are mean ± s.d., n = 3 biological independent experiments in c, e, f, i, k, l. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 5 IDA-mediated ferroptosis inhibition is independent of NRF2 and classical ferroptosis-ralated genes.
(a) ChIP-SEQ analysis of potentially regulated genes by AHR from ChIP-Altas database (http://chip-atlas.org/). (b) mRNA levels of NRF2 target genes involved in ferroptosis in HT1080 and HT29 upon IDA (50 μM) treatment. (c) Western blotting showing levels of NRF2 target genes in HT1080 and HT29 upon IDA (50 μM) treatment for the indicated time. (d) Western blot analysis of NRF2 expression in HT1080 cells expressing NRF2-sgRNA. (e) Cell viability in HT1080 cells expressing sg-ctrl or NRF2-sg treated with RSL3 and IDA (50 μM) for 24 h. (f, g) Western blot analysis of classical ferroptosis-related genes expression in HT29 (f) and MC38 (g) upon indicated concentration of IDA treatment. (h) Western blot analysis of GPX4 expression in HT1080 GPX4 KO cells. (i) Cell death of HT1080 GPX4 KO cells incubated with IDA (50 μM) with or without Fer-1 (1 μM) treatment. (j) Western blot analysis of AHR expression in HT1080 GPX4 KO cells expressing AHR-sgRNA. (k) Cell death in HT1080 GPX4 KO cells expressing AHR-sgRNA upon treatment with IDA (50 μM) or Fer-1 (1 μM). (l) Western blot analysis of GCH1 expression in HT1080 WT and GCH1 KO cells. (m) Analysis of cell death in HT1080 WT and GCH1 KO cells treated with IDA (50 μM) or RSL3 (200 nM). (n) Western blot analysis of DHODH expression in HT1080 DHODH KO cells. (o) Analysis of cell death in HT1080 WT and DHODH KO cells treated with IDA (50 μM) or RSL3 (200 nM). (p) Cell viability of HT1080 WT, FSP1 KO, GCH1 KO cells for the indicated concentrations of RSL3. (q, r) Cell viability of FSP1 KO and GCH1 KO cells upon RSL3 (200 nM) and a titration of IDA (q) or MK4 (r) treatment. The concentration of IDA is 5, 10, 20, 50, 100 μM. The concentration of MK4 is 100, 500, 1000, 2500, 5000 nM. The Western blot experiments were repeated three times independently with similar results in c, f-h, j, l and n. Data and error bars are mean ± s.d., n = 3 biological independent experiments in b, c, e, i, k, m and o-r. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 6 Supplementation with IDA does not alter the phospholipid composition.
Lipidomic profile (free fatty acids, lysophosphalipid, phosphatidylethanolamine (PE), phosphatidylcholine (PC), including plasmenyl (O) and plasmanyl (P) lipids) in HT29 cells supplemented with IDA (50 μM). Data and error bars are mean ± s.d., n = 4 independent repeats. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 7 ALDH1A3 is a transcriptional target of AHR and IDA mediated- ferroptosis inhibition is dependent of ALDH1A3.
(a, b) Volcano plots of RNA-SEQ data in HT29 cells supplemented with IDA (50 μM) (a) and IPA (50 μM) (b). (c, d) Go enrichment of IDA (50 μM) (c) treated- and IPA (50 μM) (d) treated-HT29 cells, highlighting that the pathway of NADH dehydrogenase (quinone/ubiquinone) activity is specifically enriched in IDA-treated cells. (e) Western blotting showing levels of ALDH1A3 in HT1080 upon IDA (50 μM) treatment. (f) Western blot analysis of ALDH1A3 expression in HT29 cells upon supplementation of tryptophan metabolites. Tryptophan (50 μM), IDA (50 μM), IPA (50 μM), 3-indole (50 μM), Indole-3-acetic acid (IAA, 50 μM), indole-3-aldehyde (IAld, 50 μM) and Indole-3-lactic acid (ILA, 50 μM). (g) Western blot analysis of HT29 cells expressing sg-ctrl or sg-ALDH1A3. (h) Cell viability in HT29 cells treated with RSL3, vitamin A (10 μM), retinal (10 μM) or retinoic acid (10 μM) for 24 h. (i) Western blot analysis of HT29 and HT1080 cells transfected with ALDH1A3 siRNA. (j, k) Cell death of HT29 (j) and HT1080 cells (k) expressing si-ctrl or si-ALDH1A3 treated with RSL3 and Fer-1 (1 μM) for 8 h. (l, m) Cell death of HT29 (l) and HT1080 cells (m) expressing si-ctrl or si-ALDH1A3 treated with RSL3 and IDA (50 μM) for 8 h. (n, o) Cell viability in HT29 (n) and HT1080 cells (o) expressing si-ctrl or si-ALDH1A3 treated with RSL3, vitamin A (10 μM) or retinal (10 μM) for 24 h. The Western blot experiments were repeated three times independently with similar results in e-g and i. Data and error bars are mean ± s.d., n = 3 biological independent experiments in a, b, h and j-o. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 8 ALDH1A3 promotes tumour growth in vivo by ferroptosis inhibition.
(a) Western blot analysis of ALDH1A3 expression in HT29 ALDH1A3 KO cells with ectopic expression of ALDH1A3. (b) Cell death of HT29 ALDH1A3 KO cells with ectopic expression of ALDH1A3 treated with RSL3 (5 μM) and IDA (50 μM) for 8 h. (c) Cell death of HT29 cell upon disulfiram (10 μM), RSL3 (5 μM) and Fer-1 (1 μM) for 8 h. (d) Detection of the expression of ALDH1A3 in MC38 cells transfected with shRNA for ALDH1A3. (e) Representative images of C57BL/6 J mice bearing tumours of MC38 cells transfected with indicated shRNA. n = 4 independent tumours. (f) Tumour weight of MC38 sh-ctrl or ALDH1A3-shRNA cells upon IDA treatment (50 mg/kg). n = 4 independent tumours. (g) Immunohistochemistry scoring of 4-HNE and MDA in the tumour samples from (f). Scale bars, 50 µm. n = 4 independent tumours except for n = 3 in the group of ALDH1A3-shRNA cells treated with IDA. (h) Representative images of xenograft tumours of HT29 cells treated with disulfiram (50 mg/kg) and α-Toc (10 mg/kg). (i, j) xenograft tumours (i) of HT29 cells treated with disulfiram (50 mg/kg) and α-Toc (10 mg/kg). Tumour weights were scored (j). n = 8 independent tumours. (k, l) Immunohistochemistry staining of 4-HNE (k) in the tumour samples from (i). Scale bars, 50 µm. (l) 4-HNE intensity was scored. n = 8 independent tumours. The Western blot experiments were repeated three times independently with similar results in a. Data and error bars are mean ± s.d., n = 3 biological independent experiments in b-d. All P values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 9 P.anaerobius is the major contributor of IDA biosynthesis.
(a, b) The change of mRNA levels of CYP1A1 and CYP1A2 in HT29 (a) and MC38 cells (b) upon treatment with the supernatants derived from the indicated strains. Sample 87 indicates P.anaerobius. (c) Strains that significantly upregulated cyp1a1 and cyp1a2 were subjected to detect IDA concentration by LC-MS. Sample 87 indicates P.anaerobius. (d) The relative abundance of P.anaerobius, P.russellii and C. sporogenes in CRC patients in different histological groups. Health: n = 111, MP: n = 67, Stage 0: n = 73, Stage I-II: n = 111 and Stage III-IV: n = 74.
Extended Data Fig. 10 P. anaerobius accelerates colorectal tumorigenesis via generation of IDA.
(a-e) LC-MS analysis of the contents of tryptophan(a), 3-indole(b), IAA(c), ILA(d) and IAld (e) contents derived from P. anaerobius supernatant with indicated times. n = 4 biological independent experiments. (f) Relative mRNA levels of CYP1A1 and ALDH1A3 in HT29 cells upon co-culture with E.coli or P. anaerobius. (g) Cell death of HT29 cells treated with RSL3 (5 μM) for 8 h following co-culture with E.coli or P. anaerobius. (h) Cell death of MC38 cells transfected with AHR siRNA upon ectopic expression of AHR treated with RSL3 (5 μM) for 8 h following co-culture with P. anaerobius. (i) Relative mRNA levels of CYP1A2 and ALDH1A3 in MC38 cells supplemented with supernatant from E.coli or P. anaerobius. (j) Cell death of MC38 cells treated with RSL3 (5 μM) for 8 h following supplementation with supernatant from E.coli or P. anaerobius. (k) Relative mRNA levels of CYP1A2 and ALDH1A3 in MC38 cells upon co-culture with E.coli or P. anaerobius. (l) Cell death of MC38 cells treated with RSL3 (5 μM) for 8 h following co-culture with E.coli or P. anaerobius. (m) MC38 cells-tumour bearing in C57BL/6 J mice was established and collected tumor samples 12 h after P.anaerobius intratumoral injection, followed by detecting the content of IDA in the tumor samples by LC-MS. n = 3 independent mice. (n) Cell viability of HT1080 upon RSL3 and IDA treatment. (o) Immunofluorescence staining of CD4+T, CD8+ T, macrophage (F4/80) and neutrophils (Ly6G) cells of intestinal orthotopic tumorigenesis upon IDA (i.p 50 mg/kg) treatment in AOM/DSS model of Colitis-associated cancer and positve cells was calculated. n = 3 independent mice. (p) Immunofluorescence staining of CD4+ T, CD8+ T, macrophage (F4/80) and neutrophils (Ly6G) cells in the subcutaneous tumour samples with IDA treatment (i.p,50 mg/kg) in C57BL/6 J mice and positve cells was calculated. n = 3 independent mice. Data and error bars are mean ± s.d., n = 3 biological independent experiments in f-l and n. All P values were calculated using two-tailed unpaired Student’s t-test.
Supplementary information
Supplementary Table 1
RNA-seq data for Fig. 5a.
Supplementary Table 2
RNA-seq data for Extended Data Fig. 7a.
Supplementary Table 3
RNA-seq data for Extended Data Fig. 7b.
Supplementary Table 4
LC–MS data for Fig. 6b.
Supplementary Table 5
Sequences of oligonucleotides, for example primers, RNA interference and CRISPR.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, W., Guo, M., Liu, D. et al. Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat Cell Biol 26, 124–137 (2024). https://doi.org/10.1038/s41556-023-01314-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01314-6
This article is cited by
-
Microbial regulation of ferroptosis in cancer
Nature Cell Biology (2024)