Abstract
Apoptotic cell clearance (efferocytosis) elicits an anti-inflammatory response by phagocytes, but the mechanisms that underlie this response are still being defined. Here, we uncover a chloride-sensing signalling pathway that controls both the phagocyte ‘appetite’ and its anti-inflammatory response. Efferocytosis transcriptionally altered the genes that encode the solute carrier (SLC) proteins SLC12A2 and SLC12A4. Interfering with SLC12A2 expression or function resulted in a significant increase in apoptotic corpse uptake per phagocyte, whereas the loss of SLC12A4 inhibited corpse uptake. In SLC12A2-deficient phagocytes, the canonical anti-inflammatory program was replaced by pro-inflammatory and oxidative-stress-associated gene programs. This ‘switch’ to pro-inflammatory sensing of apoptotic cells resulted from the disruption of the chloride-sensing pathway (and not due to corpse overload or poor degradation), including the chloride-sensing kinases WNK1, OSR1 and SPAK—which function upstream of SLC12A2—had a similar effect on efferocytosis. Collectively, the WNK1–OSR1–SPAK–SLC12A2/SLC12A4 chloride-sensing pathway and chloride flux in phagocytes are key modifiers of the manner in which phagocytes interpret the engulfed apoptotic corpse.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All RNA-seq data for this experiment have been submitted to the Gene Expression Omnibus under accession number GSE131860. All of the other data that support the findings of this study are available from the corresponding author on reasonable request. Source data are available online for Figs. 2–7 and Extended Data Figs. 1–7.
Code availability
The codes used for analyses are available on reasonable request.
References
Arandjelovic, S. & Ravichandran, K. S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16, 907–917 (2015).
Henson, P. M. Cell removal: efferocytosis. Annu. Rev. Cell Dev. Biol. 33, 127–144 (2017).
Gordon, S. Phagocytosis: an immunobiologic process. Immunity 44, 463–475 (2016).
Blander, J. M. The many ways tissue phagocytes respond to dying cells. Immunol. Rev. 277, 158–173 (2017).
Penberthy, K. K. & Ravichandran, K. S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 269, 44–59 (2016).
Elliott, M. R., Koster, K. M. & Murphy, P. S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 198, 1387–1394 (2017).
Green, D. R., Oguin, T. H. & Martinez, J. The clearance of dying cells: table for two. Cell Death Differ. 23, 915–926 (2016).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Canton, J., Neculai, D. & Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 13, 621–634 (2013).
Elliott, MichaelR. & Ravichandran, KodiS. The dynamics of apoptotic cell clearance. Dev. Cell 38, 147–160 (2016).
Hochreiter-Hufford, A. & Ravichandran, K. S. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 5, a008748 (2013).
Segawa, K. & Nagata, S. An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell Biol. 25, 639–650 (2015).
Levin, R., Grinstein, S. & Canton, J. The life cycle of phagosomes: formation, maturation, and resolution. Immunol. Rev. 273, 156–179 (2016).
Medina, C. B. & Ravichandran, K. S. Do not let death do us part: ‘find-me’ signals in communication between dying cells and the phagocytes. Cell Death Differ. 23, 979–989 (2016).
Morioka, S. et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563, 714–718 (2018).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017).
Morioka, S., Maueröder, C. & Ravichandran, K. S. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50, 1149–1162 (2019).
Luo, B. et al. Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity 44, 287–302 (2016).
Kim, S., Elkon, K. B. & Ma, X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21, 643–653 (2004).
Chung, E. Y. et al. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells Is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27, 952–964 (2007).
Lucas, M. et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J. Immunol. 177, 4047–4054 (2006).
Fond, A. M., Lee, C. S., Schulman, I. G., Kiss, R. S. & Ravichandran, K. S. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J. Clin. Invest. 125, 2748–2758 (2015).
Alvey, C. M. et al. SIRPA-inhibited, marrow-derived macrophages engorge, accumulate, and differentiate in antibody-targeted regression of solid tumors. Curr. Biol. 27, 2065–2077 (2017).
Shekarabi, M. et al. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 25, 285–299 (2017).
Wang, G. G. et al. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293 (2006).
Orlov, S. N., Koltsova, S. V., Kapilevich, L. V., Gusakova, S. V. & Dulin, N. O. NKCC1 and NKCC2: the pathogenetic role of cation-chloride cotransporters in hypertension. Genes Dis. 2, 186–196 (2015).
Delpire, E. & Gagnon, KennethB. E. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem. J. 409, 321–331 (2008).
Flores, B., Schornak, C., Wolfe, L., Adams, D. & Delpire, E. Functional characterization of the first known mutation of the human SLC12A2 (NKCC1) gene. FASEB J. 30, 1224.22 (2016).
Delpire, E. et al. A patient with multisystem dysfunction carries a truncation mutation in human SLC12A2, the gene encoding the Na-K-2Cl cotransporter, NKCC1. Cold Spring Harb. Mol. Case Stud. 2, a001289 (2016).
Koumangoye, R., Omer, S. & Delpire, E. Mistargeting of a truncated Na-K-2Cl cotransporter in epithelial cells. Am. J. Physiol. 315, C258–C276 (2018).
Kahle, K. T. et al. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology 21, 326–335 (2006).
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007).
Park, D., Hochreiter-Hufford, A. & Ravichandran, K. S. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 19, 346–351 (2009).
Lee, J. et al. A scaffold for signaling of Tim-4-mediated efferocytosis is formed by fibronectin. Cell Death Differ. 26, 1646–1655 (2018).
Yanagihashi, Y., Segawa, K., Maeda, R., Nabeshima, Y.-I. & Nagata, S. Mouse macrophages show different requirements for phosphatidylserine receptor Tim4 in efferocytosis. Proc. Natl Acad. Sci. USA 114, 8800–8805 (2017).
Anselmo, A. N. et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc. Natl Acad. Sci. USA 103, 10883–10888 (2006).
Vitari, AlbertoC., Deak, M., Morrice, Nick, A. & Alessi, DarioR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 391, 17–24 (2005).
Moriguchi, T. et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 280, 42685–42693 (2005).
Vitari, AlbertoC. et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem. J. 397, 223–231 (2006).
Rinehart, J. et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138, 525–536 (2009).
Zagórska, A. et al. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J. Cell Biol. 176, 89–100 (2007).
Yamada, K. et al. Small-molecule WNK inhibition regulates cardiovascular and renal function. Nat. Chem. Biol. 12, 896–898 (2016).
Arroyo, J. P., Kahle, K. T. & Gamba, G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol. Aspects Med. 34, 288–298 (2013).
Busetto, S., Trevisan, E., Decleva, E., Dri, P. & Menegazzi, R. Chloride movements in human neutrophils during phagocytosis: characterization and relationship to granule release. J. Immunol. 179, 4110–4124 (2007).
Wang, G. Chloride flux in phagocytes. Immunol. Rev. 273, 219–231 (2016).
Koncz, C. & Daugirdas, J. T. Use of MQAE for measurement of intracellular Cl− in cultured aortic smooth muscle cells. Am. J. Physiol 267, H2114–H2123 (1994).
Verkman, A. S., Sellers, M. C., Chao, A. C., Leung, T. & Ketcham, R. Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal. Biochem. 178, 355–361 (1989).
Albert, M. L., Kim, J.-I. & Birge, R. B. αvβ5 integrin recruits the CrkII–Dock180–Rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2, 899–905 (2000).
Reddien, P. W. & Horvitz, H. R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2, 131–136 (2000).
Leverrier, Y. & Ridley, A. J. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 11, 195–199 (2001).
Tosello-Trampont, A.-C., Brugnera, E. & Ravichandran, K. S. Evidence for a conserved role for CrkII and Rac in engulfment of apoptotic cells. J. Biol. Chem. 276, 13797–13802 (2001).
deBakker, C. D. et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 14, 2208–2216 (2004).
Nakaya, M., Tanaka, M., Okabe, Y., Hanayama, R. & Nagata, S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 281, 8836–8842 (2006).
Lucas, C. D. et al. Downregulation of Mcl-1 has anti-inflammatory pro-resolution effects and enhances bacterial clearance from the lung. Mucosal Immunol. 7, 857–868 (2013).
Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879 (2007).
Mishima, E. et al. Inherited, not acquired, Gitelman syndrome in a patient with Sjögren’s syndrome: importance of genetic testing to distinguish the two forms. CEN Case Rep. 6, 180–184 (2017).
Gu, X., Su, Z., Chen, M., Xu, Y. & Wang, Y. Acquired Gitelman syndrome in a primary Sjögren syndrome patient with a SLC12A3 heterozygous mutation: a case report and literature review. Nephrology 22, 652–655 (2017).
Zhou, H. et al. Complicated Gitelman syndrome and autoimmune thyroid disease: a case report with a new homozygous mutation in the SLC12A3 gene and literature review. BMC Endocr. Disord. 18, 82 (2018).
Kusuda, T. et al. Acquired Gitelman syndrome in an anti-SSA antibody-positive patient with a SLC12A3 heterozygous mutation. Intern. Med. 55, 3201–3204 (2016).
Hung, C.-M., Peng, C.-K., Wu, C.-P. & Huang, K.-L. Bumetanide attenuates acute lung injury by suppressing macrophage activation. Biochem. Pharmacol. 156, 60–67 (2018).
Acknowledgements
We thank members of the Ravichandran laboratory for discussions and reading of this manuscript. J.S.A.P. was supported by Cancer Research Institute—Mark Foundation Fellowship (NCI 1K99CA237728-01), a Burroughs Wellcome PDEP award and a NCI Cancer Research Training Award (5T32CA009109-39). S.M. is supported by grants from the Mishima-Kaiun Memorial Foundation and The Kanae Foundation for the Promotion of Medical Science. This research was supported by grants to K.S.R. from the NIGMS (GM064709), NIMH (MH096484), NHLBI (P01HL120840) and the Center for Cell Clearance at the University of Virginia, Pilot funding from the UVa Brain Institute, Odysseus I award from the FWO and an EOS grant from the FWO. E.D. is funded by NIH grants R21GM118944 and R01DK093501. C.B.M. is supported by the Pannexin Program Award through the NHLBI (P01HL120840). M.H.R. was supported by the UVA Neuroscience Training Program 4T32GM008328-25. J.I.E. is supported by NIAID Training Award 5T32AI007496. C.D.L. is supported by an award from The Wellcome Trust (206566/Z/17/Z). C.M. and B.B. have been supported by the NIH T32 Pharmacology Training Grant T32 GM00705.
Author information
Authors and Affiliations
Contributions
J.S.A.P. and S.M. designed and performed most of the experiments, with input from K.S.R., C.B.M., J.I.E., B.B., M.H.R., C.D.L., S.O.-G. and E.D. performed and/or assisted with specific experiments. J.S.A.P., S.M. and K.S.R. wrote the manuscript with input from co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SLC12A2 acts as a ‘brake’ on apoptotic cell clearance.
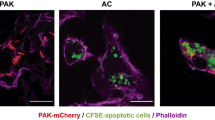
a, qPCR of Slc12a2 and Slc12a4 during efferocytosis. Data shown as mean ± SEM ***p < .001, n=4 independent experiments. b, Slc12a2 or control LR73s were fed CypHer5E-labeled apoptotic Jurkat cells for 2h. Data are from n=6 independent experiments. Data shown as mean ± SEM. ***p < .001. Validation of Slc12a2 deletion by qPCR for two independent small guides. c, (Left panel) Slc12a2 siRNA-treated LR73 phagocytes were incubated with CypHer5E-labeled apoptotic Jurkat cells for 2h. Data represent n=3 independent experiments. Data shown as mean ± SEM. *p < .05, ns = not significant. (Right panel) siRNA targeting efficiency of Slc12a2 in LR73 phagocytes via qPCR. ***p < .001. d,e (d) LR73 phagocytes or (e) peritoneal macrophages were pre-treated for 1h with bumetanide then incubated with CypHer5E-labeled apoptotic Jurkat cells for 2h (e), or 1h f, Data represent n=3 (d, ***p < .001, mean ± SEM) or n=2 independent experiments (e). (f) Control or Slc12a2-deficient LR73 phagocytes were pre-treated for 1h with bumetanide then incubated with CypHer5E-labeled apoptotic Jurkat cells for 2h. Data shown as mean ± SD, n=3 independent experiments. ns = not significant. g, Control or Slc12a2-deficient LR73 phagocytes were cultured with apoptotic cells, with or without Annexin V for 2h. Data are n=3 independent experiments. Data shown as mean ± SEM. ***p < .001. h, qPCR determination of the knockdown efficiency of Slc12a2 in ER-Hoxb8 immortalized bone marrow-derived macrophages used in Fig. 2b. n=2 independent experiments. i, Mice were injected i.p. with the SLC12A2 inhibitor bumetanide. After 1h, CypHer5E-labeled apoptotic Jurkat cells were injected. Peritoneal cells were collected and engulfment by CD11b+ F4/80+ macrophages assessed by flow cytometry. Data represent n=2 independent experiments. j, SLC12A2-deficient macrophages were cultured for 3h with apoptotic cells prior to imaging. Efferocytosis was assessed by fluorescent microscopy. Images represent four independent experiments. scale bar= 20 microns. Statistics source data are provided in Source Data Extended Data Fig. 1.
Extended Data Fig. 2 SLC12A4 acts as an ‘accelerator’ during apoptotic cell clearance.
a, LR73 phagocytes were incubated with apoptotic Jurkat cells for the indicated times. Apoptotic cells were thoroughly washed away with cold PBS, and total LR73 cell lysate was collected on ice and analyzed by immunoblotting using the indicated antibodies and the band intensities were quantitated. After normalization to ERK (control protein for each sample), the fold changes in band intensity relative to the 0 min time point are indicated. b, (Left) LR73 cells with CRISPR/Cas9-mediated deletion of Slc12a4 (or control cells with Cas9-GFP but scramble guide RNAs) were co-cultured with CypHer5E-labeled apoptotic Jurkat cells for 2 h, and efferocytosis assessed. Data from n=2 independent experiments with 2 unique clones per condition (n=4 independent experiments). Data shown as mean ± SEM. ***p < .001. (Right) Also shown are the knockdown efficiencies of two small guide RNA used to target SLC12A4. c, LR73 cells were co-cultured with apoptotic cells for 2 h or 8 h and assessed for efferocytosis by flow cytometry. Data are from n=4 independent experiments. Data shown as mean ± SEM. ***p < .001. d, (Left) LR73 phagocytes were transfected with siRNAs targeting Slc12a4 and incubated with CypHer5E-labeled apoptotic Jurkat cells for 2 h. ***p < .001. Data represent n=3 independent experiments and are shown as mean ± SEM. (Right) qPCR of siRNA-mediated Slc12a4 knockdown efficiency in LR73 phagocytes. ***p < .001. e, Similar to (d), but for 2 h and 8 h. Data are representative of n=4 independent experiments and are shown as mean ± SEM. ***p < .001. f, Control or Slc12a2-deficient phagocytes were treated with siRNA targeting Slc12a4 or control siRNA for 48h and incubated with apoptotic cells for 2 h. Data represent n=3 independent experiments. ns = not significant, ***p < .001. Statistics source data are provided in Source Data Extended Data Fig. 2. Unprocessed blots are provided in Unprocessed Blots Extended Data Fig. 3.
Extended Data Fig. 3 SLC12A2 deficiency overrides homeostatic efferocytosis signature.
a,b, Overexpression of SLC12A2 restores appetite suppression and reverses the pro-inflammatory gene signature in engulfing Slc12a2-deficient phagocytes. A control vector or Slc12a2 cDNA was overexpressed in control or Slc12a2-deficient LR73 phagocytes. Phagocytes were then mixed with CypHer5E-labeled apoptotic Jurkat cells for 2 h, cDNA+ CypHer5E+ phagocytes were sorted (a) and assessed for the presence of a pro-inflammatory gene signature by qPCR (b). Data represent n=3 independent experiments. Data shown as mean ± SEM. ns = not significant, ***p < .001. c, Slc12a2 knockdown induces pro-inflammatory genes in normally anti-inflammatory efferocytosis after TIM-4 overexpression. TIM4 stably expressing LR73 phagocytes were transfected with control siRNA or Slc12a2 siRNA, and then co-cultured with apoptotic cells for 2 h. RNA was subsequently isolated from the phagocytes, and the pro-inflammatory gene signature was assessed via qPCR. Data represent n=3 independent experiments. Data shown as mean ± SEM. ns = not significant, ***p < .001. Statistics source data are provided in Source Data Extended Data Fig. 3.
Extended Data Fig. 4 WNK1, OSR1, & SPAK deficiency phenocopies SLC12A2 during apoptotic cell clearance.
a, Phosphorylation of SLC12A2 and SLC12A4 during efferocytosis. LR73 phagocytes were mixed with apoptotic Jurkat cells for the indicated times, and apoptotic cells thoroughly washed. Total lysates of LR73 cells were analyzed using phosphate affinity electrophoresis (see diagram). 12A2-P and 12A4-P denote the phospho-mobility shift, while 12A2 and 12A4 denote the non-phosphorylated forms. Quantification was based on the intensity and size of the bands followed by subtracting background value. Fold change relative to time point 0 is shown after normalization to ERK2 loading control. Molecular size marker does not show their aciculate sizes due to the modified gels. b,c, Validation of Oxsr1 or Stk39 knockdown in LR73 phagocytes. Knockdown efficiency of siRNAs targeting Oxsr1 (OSR1) or (C) Stk39 (SPAK) were determined by qPCR. ***p < .001, n=3 (Stk39) or 4 (Osr1) independent experiments, mean ± SEM. d, siRNA targeting of Wnk1 results in increased efferocytosis. (Left) LR73 phagocytes were transfected with siRNAs targeting Wnk1 and incubated with CypHer5E-labeled apoptotic Jurkat cells for 2 h. Data are presented as phagocytosis index and represent n=4 independent experiments. Data shown as mean ± SEM. (Right) Wnk1 knockdown efficiency of two independent siRNAs, determined by qPCR. ***p < .001. e, Wnk1 deficiency phenocopies Slc12a2 deficiency in phagocytes during efferocytosis. GFP+ Wnk1-deficient LR73 cells were co-cultured with CypHer5E-labeled apoptotic Jurkat cells for 2 h. ***p < .001. Data are from n=3 independent experiments. Data shown as mean ± SEM. f, qPCR validation of CRISPR/Cas9-mediated targeting of Wnk1 in LR73 phagocytes. *p < .001. The drop in mRNA expression due to individual small guide used to target Wnk1 are shown. Statistics source data are provided in Source Data Extended Data Fig. 4. Unprocessed blots are provided in Unprocessed Blots Extended Data Fig. 3.
Extended Data Fig. 5 SLC12 pathway regulates cell size of efferocytic phagocytes.
a, From experiments in Fig. S1b, average Forward Scatter – Area (FSC-A) of 10,000 phagocytes was determined and presented as change in size of Slc12a2-deficient cells relative to control LR73 cells, with or without apoptotic cells. Data represent analysis of at least 2,000 engulfing (CypHer5E+) events from n=4 independent experiments. Data shown as mean ± SEM. ***p < .001. b, Similar to (a), cell size analysis via FSC-A from experiments in Fig. 2c. Data represent n=3 independent experiments. Data shown as mean ± SEM. ***p < .001. c, Similar to (a), cell size analysis via FSC-A from experiments in Fig. 2b). Data represent n=4 independent experiments. Data shown as mean ± SEM. * p < .05, ***p < .001. d, Similar to (a), cell size analysis via FSC-A from experiments in Supplementary Fig. S4e. Data represent n=4 independent experiments with 2 biological replicates per condition. Data shown as mean ± SEM. ***p < .001. e,f, LR73 phagocytes overexpressing the PtdSer receptor TIM4 maintain their cell size while engulfing more apoptotic cells. LR73 phagocytes transfected with control or Timd4 (TIM4) plasmid were incubated with CypHer5E-labeled apoptotic Jurkat cells for 2h and their efferocytosis (f) and cell size by FSC-A g, assessed. Data represent n=3 independent experiments. Data shown as mean ± SEM. ***p < .001. ns = not significant. (g) Similar to (a), cell size analysis via FSC-A from experiments in Supplementary Fig. S2b. Data from n=4 independent experiments. h, Chloride ionophore TBTC reverses cell size increase in Slc12a2-deficient phagocytes. Average cell size was quantified by FSC-A from the experiments performed in Fig. 6a. ns = not significant. Data shown as mean ± SEM, n=4 independent experiments. Statistics source data are provided in Source Data Extended Data Fig. 5.
Extended Data Fig. 6 Chloride flux during efferocytosis.
a, LR73 phagocytes were labeled with MQAE for 2h, washed, treated with tributyltin chloride (TBTC), a chloride ionophore. Treatment with TBTC results in a significant quenching/decrease of MQAE fluorescence due to greater chloride levels. Data represent two independent experiments. b, Chloride flux during efferocytosis monitored by MQAE. Performed as in Fig. 6c. Shown are representative plots of MQAE+ phagocytes cultured without or with apoptotic cells. Gates indicates phagocytes actively engulfing apoptotic cells (CypHer5Ehigh), displaying relatively low chloride levels (MQAEbright). c, Slc12a2- and Slc12a4-deficient phagocytes have comparable MQAE signal prior to efferocytosis. From experiments in Fig. 6d, e. LR73 phagocytes treated with control, Slc12a4, or Slc12a2 siRNA were labeled with MQAE and the basal MQAE plots without incubation with apoptotic cells are shown. d, TIM4 overexpression leaves chloride flux unaffected. LR73 phagocytes transfected with control or Timd4 (TIM4) plasmid were labeled with MQAE and incubated with CypHer5E-labeled apoptotic thymocytes for 2h and assessed for chloride flux. Data represent n=3 independent experiments. ns = not significant. e,f, Macrophages from Slc12a2mut mice show decreased chloride influx during apoptotic cell clearance. Bone marrow-derived macrophages from Slc12a2mut mice or control littermates were labeled with MQAE and incubated with apoptotic thymocytes for 1h. Data represent n=3 independent experiments. ***p < .001. g,h, WNK1, OXSR1, and SPAK contribute to influx of extracellular chloride during efferocytosis. Wnk1, Oxsr1 (OSR1), and Stk39 (SPAK) were targeted by siRNA and analyzed as in Fig. 6e above (g). A summary plot of CypHer5E+ MQAE “bright” phagocytes is shown (h). Data represent n=3 independent experiments, shown as mean ± SEM. ***p < .001. i, WNK1, OSR1, and SPAK have comparable basal MQAE signal prior to efferocytosis. Controls for Fig. S6g and S6h, showing basal MQAE signal from LR73 cells incubated without apoptotic cells. Data represent three independent experiments. Statistics source data are provided in Source Data Extended Data Fig. 6.
Extended Data Fig. 7 SLC12 pathway regulates ROS levels and pro-inflammatory cytokine production during efferocytosis.
a, Inhibition of SLC12 pathway during efferocytosis results in increased pro-inflammatory cytokine production by thymic macrophages ex vivo. Performed as in Fig. 7d, except thymic macrophages were isolated and cultured for 6 h, then supernatants were collected and analyzed for pro-inflammatory cytokine production. Data are representative of n=2 independent experiments. b, Inhibition of SLC12 pathway induces accumulation of ROS in efferocytic phagocytes. Data are from Fig. 7c, but instead normalized to vehicle + no AC control to demonstrate parity between control and WNK463-treated phagocytes not exposed to ACs, n=4. Data shown as mean ± SEM. **p < .01, ***p < .001, ns = not significant. Statistics source data are provided in Source Data Extended Data Fig. 7.
Supplementary information
Supplementary Tables
Supplementary Table 1: cell volume associated genes. Listed are members of the SLC12 (electroneutral chloride transporter) pathway genes with altered expression (based on adjusted P value and log2-transformed fold change as determined by DESeq2) after corpse internalization, but not due to soluble factors or corpse contact. Supplementary Table 2: Anti- and Pro-Inflammatory genes list of genes associated with autoimmunity and chronic inflammatory disease that arose from Slc12a2-deficient efferocytic phagocytes (Fig. 4). Supplementary Table 3: qPCR TaqMan probes list of all of the hamster and mouse TaqMan probes used.
Supplementary Video 1
Control ER-Hoxb8 BMDMs were cultured for 3 h with apoptotic cells labeled with CypHer5E before imaging. Apoptotic cells were then washed away and actively engulfing phagocytes, as determined by microscopy, were imaged for the loss of CypHer5E signal over time. All videos are over a 5 h time course with frame intervals of 10 min. The videos are representative of two independent experiments with two replicates per condition.
Supplementary Video 2
Control ER-Hoxb8 BMDMs were cultured for 3 h with apoptotic cells labeled with CypHer5E before imaging. Apoptotic cells were then washed away and actively engulfing phagocytes, as determined by microscopy, were imaged for the loss of CypHer5E signal over time. All videos are over a 5 h time course with frame intervals of 10 min. The videos are representative of two independent experiments with two replicates per condition.
Supplementary Video 3
Control ER-Hoxb8 BMDMs were cultured for 3 h with apoptotic cells labeled with CypHer5E before imaging. Apoptotic cells were then washed away and actively engulfing phagocytes, as determined by microscopy, were imaged for the loss of CypHer5E signal over time. All videos are over a 5 h time course with frame intervals of 10 min. The videos are representative of two independent experiments with two replicates per condition.
Supplementary Video 4
Slc12a2-deficient ER-Hoxb8 BMDMs were cultured for 3 h with apoptotic cells labeled with CypHer5E before imaging. Apoptotic cells were then washed away and actively engulfing phagocytes, as determined by microscopy, were imaged for the loss of CypHer5E signal over time. All videos are over a 5 h time course with frame intervals of 10 min. The videos are representative of two independent experiments with two replicates per condition.
Supplementary Video 5
Slc12a2-deficient ER-Hoxb8 BMDMs were cultured for 3 h with apoptotic cells labeled with CypHer5E before imaging. Apoptotic cells were then washed away and actively engulfing phagocytes, as determined by microscopy, were imaged for the loss of CypHer5E signal over time. All videos are over a 5 h time course with frame intervals of 10 min. The videos are representative of two independent experiments with two replicates per condition.
Supplementary Video 6
Slc12a2-deficient ER-Hoxb8 BMDMs were cultured for 3 h with apoptotic cells labeled with CypHer5E before imaging. Apoptotic cells were then washed away and actively engulfing phagocytes, as determined by microscopy, were imaged for the loss of CypHer5E signal over time. All videos are over a 5 h time course with frame intervals of 10 min. The videos are representative of two independent experiments with two replicates per condition.
Source data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 2
Unprocessed Western Blots
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 4
Unprocessed Western Blots
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Rights and permissions
About this article
Cite this article
Perry, J.S.A., Morioka, S., Medina, C.B. et al. Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat Cell Biol 21, 1532–1543 (2019). https://doi.org/10.1038/s41556-019-0431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0431-1
This article is cited by
-
Drugging the efferocytosis process: concepts and opportunities
Nature Reviews Drug Discovery (2022)
-
A genome-wide CRISPR screen identifies WDFY3 as a regulator of macrophage efferocytosis
Nature Communications (2022)
-
Altered function and differentiation of age-associated B cells contribute to the female bias in lupus mice
Nature Communications (2021)
-
Chloride sensing by WNK1 regulates NLRP3 inflammasome activation and pyroptosis
Nature Communications (2021)
-
Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV
Nature (2021)