Abstract
The enantioselective synthesis of S-stereogenic sulfinamides has garnered considerable attention due to their structural and physicochemical properties. However, catalytic asymmetric synthesis of sulfinamides still remains daunting challenges, impeding their broad application in drug discovery and development. Here, we present an approach for the synthesis of S-stereogenic sulfinamides through peptide-mimic phosphonium salt-catalyzed asymmetric skeletal reorganization of simple prochiral and/or racemic sulfoximines. This methodology allows for the facile access to a diverse array of substituted sulfinamides with excellent enantioselectivities, accommodating various substituent patterns through desymmetrization or parallel kinetic resolution process. Mechanistic experiments, coupled with density functional theory calculations, clarify a stepwise pathway involving ring-opening and ring-closing processes, with the ring-opening step identified as crucial for achieving stereoselective control. Given the prevalence of S-stereogenic centers in pharmaceuticals, we anticipate that this protocol will enhance the efficient and precise synthesis of relevant chiral molecules and their analogs, thereby contributing to advancements in drug discovery.
Similar content being viewed by others
Introduction
Over the past century, innovations of catalytic asymmetric synthesis have rapidly expanded the realm of accessible chiral chemical entities for pharmaceutical research1,2,3. Despite some impressive advances have been achieved, the breadth and depth of chiral skeletal diversification in discovery and development of new drugs are still constrained due to the unsolved problems in particularly important catalytic asymmetric transformations4. In this scenario, a conspicuous example is that the sporadic occurrence of catalytic protocols towards chiral sulfinamides, a class of stereogenic-at-S(IV) scaffolds, which is thus difficult to fulfill the demands of systematically biological screening (Fig. 1a)5,6,7,8,9,10,11,12,13,14. To date, the most conventional and widely employed approach for synthesizing chiral sulfinamides primarily relies on using chiral starting materials15,16,17 or employing stoichiometric amounts of chiral auxiliaries18,19. Furthermore, catalytic strategies involve kinetic resolution enabled by enzyme9 or transition metal20 and asymmetric oxidation10. As such, a reliable and efficient method that will enable the construction of structurally and stereochemically diverse sulfinamide targets to meet diversified demands21,22 from multiple disciplines is highly desirable and urgently required.
a Importance of chiral/achiral sulfinamide compounds. b Challenge for sulfinamide chemistry: no precedent for transformation of sulfoximines to sulfinamides in a catalytic enantioselective manner. c This work: PPS-catalyzed enantioselective skeletal reorganization of sulfoximines for unified access to chiral sulfinamides.
Organocatalytic asymmetric reduction of hexavalent sulfoximines to sulfinamides represents an attractive and direct solution towards above target-specific synthesis. However, despite the considerable research efforts that have been focused on this area, compared to the well-established and widely-applied transformations of sulfinamides to sulfoximines, the concurrent S = O and S = N bonds in sulfoximine skeletons typically pose an inherent chemoselectivity challenge for such reduction process, let along the challenging ambiguous stereo-control, and thus no successful example has been developed so far (Fig. 1b, left)23. Of note, such type of reduction process was pioneered almost 30 years ago by Mock24, Gais25 and Pyne26, respectively via a skeletal reorganization strategy. However, their approaches had limited substrate scope and yielded moderate chemical yields. Despite the intriguing mechanism and high atom-economy27,28 associated with skeletal reorganization, it is surprising that a catalytic asymmetric version of this reaction has not been realized to date. The major challenges encountered in such asymmetric skeletal reorganizations not only lie in the high barrier in the S-C bond cleavage, which generally necessitates expensive transition-metal catalysts together with high reaction temperatures, undoubtedly limiting its broad application and functional group tolerance29, but also come from the formidable stereo-differentiation in the indistinguishable S-, O-, and N-nucleophilic centers of the in situ generated sulfinamide anions30,31,32,33,34,35,36. In addition, such process towards the target products might proceed with unpopular desulfurization reaction26.
Recognizing the notable obstacles presented in the aforementioned protocol, and to unlock the synthetic and biological potential of yet underutilized sulfinamide scaffolds, we sought to realize the catalytic enantioselective skeletal reorganization of achiral sulfoximines, thus producing the important chiral sulfinamide scaffolds. If successful, this proposed approach would establish a versatile molecular platform for the synthesis of libraries containing enantioenriched sulfinamide compounds, and potentially, for the generation of value-added downstream products (Fig. 1b, right)7,37,38,39. Inspired by our recent disclosure of peptide-mimic phosphonium salt (PPS) catalysts and their wide applications in asymmetric synthesis40,41,42,43,44,45, we anticipated that the enhanced ion-pairing induction and multiple hydrogen-bonding interaction of PPS catalyst system could potentially provide synergistic activation for promoting this transformation reaction bearing much less reactivity of substrates. Furthermore, this class of conformationally flexible and highly structural tunable catalysts might be also suitable candidates for addressing the major stereo-control challenge of such enantioselective skeletal reorganization reaction.
Herein, we disclose a PPS-catalyzed enantioselective skeletal reorganization of sulfoximines, guiding the precise and highly efficient construction of optically pure S-stereogenic sulfinamides (Fig. 1c). Crucially, the use of PPS catalysts bypass both the transition-metal catalysts and the previous high reaction temperatures required by the in-situ generation of conjugated diene-tethered sulfinamide intermediates, in which the existence of conjugation element may ensure an effective thermodynamic driving force for the initial enantioselective S-C bond cleavage. Moreover, this protocol employing prochiral and/or racemic cyclic sulfoximines as starting reagents and harnessing one-pot operations with cascade process leads to a broad range of enantioenriched cyclic sulfinamides with an assortment of functional groups in high yields with excellent enantioselectivities.
Results
Reaction development
Given the aforementioned challenges, we initiated our investigations by testing the skeletal reorganization of prochiral cyclic sulfoximine A1 with a free NH unit as a benchmark reaction in the presence of a racemic PPS catalyst P2 (Fig. 2a). However, no formation of the desired sulfinamide was observed, which might be attributed to the NH-involving hydrogen transfer process. Thus, we prepared a series of N-protected cyclic sulfoximines and tested the validity of our hypothesis. Encouragingly, the use of the naphthyl protecting group could afford the desired skeletal reorganization product with a nearly quantitative yield, while the other N-protected sulfoximines A2-A4 suffered from the instability problem. Control experiments uncovered that the nature of ion-pairing and H-bonding effects appeared to play a pivotal role in realizing this reaction, wherein the lack of H-bonding donors (Fig. 2b, entry 1), let alone the lack of PPS catalysts (Fig. 2b, entry 3), would result in a sluggish reaction under otherwise identical conditions. Additionally, the loss of reactivity without the base also indicated an ionic reaction pathway (Fig. 2b, entry 4).
Encouraged by the preliminary success, we turned our attention to the sought-after catalytic enantioselective skeletal reorganization of prochiral cyclic sulfoximines. As shown in Fig. 3a, initial extensive evaluation of chiral PPS catalysts revealed that the (L, L)-dipeptide-based phosphonium salt catalyst (P1) led to inspiring enantioinduction, albeit with a decrease in the reactivity. Exchanging the methyl substituent of the P(V) atom to a benzyl group could enhance the reactivity. As such, further skeleton optimization of PPS catalysts was performed, wherein L-tert-Leu-L-tert-Leu-derived phosphonium salt P4 furnished the product 1 in 92% yield with 28% e.e. In view of the density functional theory (DFT) calculated electrostatic potential (ESP) of chiral PPS cationic catalyst P8 (Fig. 3c), we speculated that multiple hydrogen-bonding donors with an improved electropositive region could offer better recognition of the transition state structures. Thus, the substituent effects of the active P(V)-center were investigated, and surprisingly, catalyst P8 led to a significant increase in enantioselectivity (82% e.e.). Then, the catalyst P8 was used for further screening of other parameters (see the Supplementary Tables 3–8 for details). Remarkably, an obvious increase in enantioselectivity (i.e. 95% e.e.) coupled with a satisfactory yield was observed while lowering the reaction temperature to −40 °C, even under a very low catalyst loading of 5 mol% (Fig. 3b).
a Catalyst screening. b Effects of other reaction parameters including temperature and catalyst loading. c Electrostatic potential surface (ESP) of cationic catalyst P8. Unless indicated, all the reactions were performed with A5 (0.1 mmol), P (20 mol%), KOH (2.0 equiv.), in toluene (2.5 ml) for 6 hours. aThe reaction was stirred for 24 h. bP8 (5 mol%) was used and the reaction was stirred for 4 days. The isolated yields were given. All the e.e. values were determined by HPLC analysis.
Reaction scope
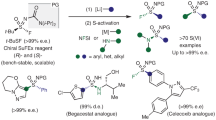
With the optimal catalyst and other reaction conditions in hand, the substrate scope of this catalytic asymmetric skeletal reorganization was systematically explored. As shown in Fig. 4, a variety of symmetric cyclic sulfoximines with aryl substitutions on the alkene moiety were firstly prepared and subjected to this transformation. Obviously, both electron-donating and electron-withdrawing groups at different positions (i.e. para-, meta- or ortho-position) were tolerated well, affording the corresponding products (1−9) in good to excellent yields (70−99%) with good enantioselectivities (90−96%). Likewise, disubstituted substrates(A14–A16) were also suitable for this transformation. To further expand the generality of this protocol, other orthogonal N-protecting groups were examined. Good functional group compatibility was, in most cases, observed with tolerance of a series of sterically and electronically differentiated aromatics and heteroaromatics (A17−A43). Besides, a decrease in stereocontrol of sulfinamides 19 and 25 indicated the importance of steric hindrance of the aromatic ring bonded to nitrogen atom at the ortho-position. Of note, this reaction could be scalable without any loss of efficiency (eg. 1 and 17). Additionally, the absolute configurations of 12, 16 and 20 were determined by X-ray diffraction analysis and those of other products were assigned by analogy. Remarkably, beyond symmetric cyclic sulfoximines, this catalytic system was also effective for non-symmetric counterparts. For example, the racemic sulfoximines bearing different aryl groups on the C = C moiety could be engaged to this skeletal reorganization reaction, simultaneously yielding two classes of expected enantioenriched products (such as 40/41 and 42/43), absolute configurations of which were confirmed by X-ray crystallographic analysis. This sophisticated parallel kinetic resolution46 emphasized the practicability of this construction strategy towards chiral sulfinamide compounds.
Standard reaction conditions: A (0.1 mmol), P8 (10 mol%), KOH (2.0 equiv.) in toluene (1.0 ml) at −40 oC for 6 hours. aThe reaction was stirred for 48 hours. bReaction was performed on a 2.5 mmol scale. cReaction was performed on a 1.1 mmol scale. The isolated yields were given. dAfter once recrystallization. The e.e. values were determined by HPLC analysis.
Synthetic applications
To investigate the versatility of this catalytic system, we subsequently examined its application in the late-stage functionalization of complex skeletons. To our delight, all of the elaborate sulfoximines derived from bioactive molecules or material building blocks, such as Gemfibrozil, Stearic acid, Linoleic acid etc., could serve as effective substrates, furnishing the corresponding skeletal reorganization products (44−47) in good yields with excellent enantioselectivities (Fig. 5a). Furthermore, a wide variety of powerful synthetic transformations of the resulting chiral sulfinamides were also demonstrated. For instance, the sulfinamide 1 could be readily converted into sulfonimidamide 48 and sulfonamide 49 by simple amination and oxidation. Of note, an unexpected desulfurized aromatization occurred towards the facile assembly of the pyrrole skeleton 50 under the transition metal catalytic condition (Fig. 5b, left). Another interesting and important application would be the expedient installation of various types of functionalities via a series of cross-coupling (i.e. Miyaura borylation, Buchwald-Hartwig coupling and Suzuki coupling) of brominated chiral sulfinamides, affording the corresponding products (51−53) without any loss of enantiopurity (Fig. 5b, right). Notably, the vulnerable nature of the S−N bond in sulfinamides rendered them versatile building blocks for further elaborations towards chiral sulfoxide compounds (54−58), many of which have been previously deemed as synthetically challenging (Fig. 5b, bottom)47,48. Notably, though a striking steric-hindrance for better asymmetric induction has been demanded, the challenging N-alkyl-substituted sulfoximine also gave access to the corresponding skeletal reorganization product in 85% isolated yield with 80% e.e. and 10:1 d.r. via one-pot operation, employing an improved phosphonium salt catalyst (Fig. 5c).
a Late-stage diversification of bioactive molecules and material building blocks. b Derivatization of the chiral sulfinamide products. I, Amination. Sulfinamide 1 (0.1 mmol), ammonium carbamate (2.0 equiv.) and iodobenzene diacetate (2.5 equiv.) in MeOH at r.t. II, Oxidation. Sulfinamide 1 (0.1 mmol), mCPBA (1.4 equiv.) and NaHCO3 (2.5 equiv.) in CH2Cl2 at r.t. III, Aromatization. Sulfinamide 1 (0.1 mmol), Pd(OAc)2 (10 mol%), XPhos (30 mol%) and Cs2CO3 (1.4 equiv.) in dioxane at 100 oC. IV, SN2 substitution. Sulfinamide (0.1 mmol) and ArMgCl (1.2 equiv.) in CPME solvent at −80 oC. V, Suzuki Coupling. Sulfinamide 17 (0.1 mmol), aryl boronic acid pinacol ester (1.2 equiv.), Pd(PPh3)4 (5 mol%) and K2CO3 (2.0 equiv.) in THF/H2O (v/v = 3/1) at 90 oC. VI, Buchwald-Hartwig Cross Coupling. Sulfinamide 17 (0.1 mmol), BocNH2 (1.5 equiv.), Pd(OAc)2 (10 mol%), XPhos (30 mol%) and Cs2CO3 (1.4 equiv.) in dioxane at 100 oC. VII, Miyaura Borylation. Sulfinamide 17 (0.1 mmol), Pd(dppf)Cl2 (10 mol%), B2pin2 (4.0 equiv.) and KOAc (5.0 equiv.) in dioxane at 90 oC. Also see the synthetic application section in Supplementary Information for more condition details. c Approach to N-alkyl sulfinamide via one-pot operation.
Mechanistic investigations
To shed light on this underlying reaction mechanism, a series of kinetic and spectroscopic studies were performed. Our initial interest was focused on the identification of the key intermediate in this organocatalytic skeletal reorganization of sulfoximines. Thus, a time-course study for this reaction process with substrate A5 was conducted (Fig. 6a). The kinetic profile indicated the presence of a short-lived enantioenriched species, which was confirmed as the conjugated diene-tethered sulfinamide B. Additionally, we performed several elaborate control experiments. As shown in Fig. 6b, such chiral specie B could be smoothly converted into the desired chiral product 1 without erosion of enantioselectivity, irrespective of harnessing either chiral catalyst P8 or racemic catalyst P2. Based on these results, we suggested an enantioselective ring-opening and subsequent ring-closing step-wise pathway towards the facile access to these chiral sulfinamide products.
Subsequently, we turned our interest in stereochemical probing to the significant ring-opening step. The nonlinear effect experiment between chiral catalyst P8 and product 1 was firstly conducted (Fig. 6c), and at last a linear relationship was observed, which uncovered a monomeric catalytic mode in the stereo-determining step, in correspondence with the Job Plot analysis of the 1H NMR titration experiments (see the Supplementary Figs. 16–18 for details). Further kinetic studies also illustrated that the overall reaction is the first order relationship with the catalyst P8 (Fig. 6d). Then, to investigate the nature of multiple weak-bonding activations, the methylated phosphonium salts P8-1 and P8-2 were prepared and subjected to the standard reaction (Fig. 6e). As a result, both of the reactivity and enantioselectivity suffered from a dramatical decline, thus indicating the indispensability of H-bonding interactions in the asymmetric induction.
To provide further molecular-level insight into this process, DFT calculations were carried out to understand the reaction pathway and the origin of stereoselectivity by employing Gaussian 09 program package (see the Supplementary Information for more details)49. As shown in Fig. 7, the catalyst cation P8 firstly interacted with the oxygen atom of the deprotonated substrate via dual H-bonding interactions, forming the intermediates IM-B1-R and IM-B1-S, alternatively. The NH···O(S) distances in IM-B1-R were 1.96 and 1.86 Å, which were slightly shorter than those in IM-B1-S (2.10 and 1.87 Å). Accordingly, IM-B1-R was more stable than IM-B1-S by 2.1 kcal mol−1. Then, IM-B1-R and IM-B1-S underwent the ring-opening process, affording IM-B2-R and IM-B2-S via transition states TS1-R and TS1-S, respectively. Due to significant repulsion between ortho-CH3 group in the Bn unit of catalyst and the Ph group of sulfoximine substrate, as well as unfavorable steric effect between the two t-Bu groups and the naphthyl group, the relative Gibbs free energy (∆G) of TS1-R was lower than that of TS1-S by 1.7 kcal mol−1. Next, IM-B2-R and IM-B2-S underwent C−S single bond rotation, followed by ring-closing process to construct the C−N bonds in the IM-B4-R and IM-B4-S. This step could occur easily, with the ΔG≠ as low as 4.6 and 4.2 kcal mol−1, respectively. Otherwise, the energy profile for transformation of conjugated diene-tethered anion to sulfinamide anion along R-path without catalyst has also been calculated, which was highly consistent with the experimental results (Fig. 6) and reasonably illustrated that the “N”-selective ring-closing might be a stereospecific process to form the product with R-configuration eventually. In the view point of energy, the ring-opening of the sulfoximine was the key step of stereocontrol with the ΔΔG of 1.7 kcal mol−1. The theoretical stereoselectivity was predicted to be 94% e.e. at 233 K, which was close to experimental observation (95% e.e.).
Discussion
In summary, we have successfully developed the catalytic enantioselective skeletal reorganization of sulfoximines, facilitating the creation of enantioenriched sulfinamides featuring a S-stereocenter. Amid this process, peptide-mimic phosphonium salts, which serve as multifunctional weak-bonding catalysts to not only activate C−S bonds but also afford efficient asymmetric induction, are crucial for this formal metal-free reduction. Remarkably, leveraging the versatility of this class of cyclic sulfinamides, this current methodology could provide a powerful platform to allow access to a wide variety of sought-after stereogenic-at-sulfur scaffolds, such as chiral sulfonimidamides and sulfoxides etc. We anticipate that this general and efficient method for the synthesis of enantioenriched sulfinamide compounds would open up an avenue for related drug discovery and development.
Methods
Procedure for the synthesis of chiral sulfinamides 1−47
Sulfoximines A (0.1 mmol), KOH (0.2 mmol, 2.0 equiv.) and catalyst P8 (0.01 mmol, 10 mol%) were added to a dried reaction tube with a magnetic stirring bar under air, followed by the addition of precooling toluene (1.0 mL). The reaction mixture was stirred at −40 °C until completion determined by TLC. Then, the reaction mixture was directly purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1) to afford the desired products.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2115196 (P6), CCDC 2235143 (12), CCDC 2210348 (16), CCDC 2213077 (20), CCDC 2262526 (41), CCDC 2265808 (42) and CCDC 2244448 (48). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All information relating to initial studies, optimization studies, experimental procedures, mechanistic studies, DFT calculations, high-performance liquid chromatography spectra, NMR spectra, high-resolution mass spectrometry and optical rotation data are available in Supplementary Information. All other data are present and available from the corresponding authors upon request. Source data are provided with this paper.
References
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C-H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Rodríguez, P. N., Ghashghaei, O., Bagán, A., Escolano, C. & Lavilla, R. Heterocycle-based multicomponent reactions in drug discovery: from hit finding to rational design. Biomedicines 10, 1488–1506 (2022).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Tilby, M. J. & Willis, M. C. How do we address neglected sulfur pharmacophores in drug discovery? Expert Opin. Drug Discov. 16, 1227–1231 (2021).
Scott, K. A. & Njardarson, J. T. Analysis of US FDA‑approved drugs containing sulfur atoms. Top. Curr. Chem. 376, 5 (2018).
Feng, M., Tang, B., Liang, S. H. & Jiang, X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 16, 1200–1216 (2016).
Zhang, X., Wang, F. & Tan, C.-H. Asymmetric synthesis of S(IV) and S(VI) stereogenic centers. JACS Au 3, 700–714 (2023).
Savile, C. K., Magloire, V. P. & Kazlauskas, R. J. Subtilisin-catalyzed resolution of N-Acyl Arylsulfinamides. J. Am. Chem. Soc. 127, 2104–2113 (2005).
Ma, L.-J. et al. Chiral Brønsted-acid-catalyzed asymmetric oxidation of sulfenamide by using H2O2: a versatile access to sulfinamide and sulfoxide with high enantioselectivity. ACS Catal. 9, 1525–1530 (2019).
Zheng, G.-L., Lu, C., Cheng, J.-P. & Li, X. Kinetic resolution of sulfinamides via asymmetric N‑allylic alkylation. Org. Lett. 23, 8499–8504 (2021).
Wen, Q., Zhang, L., Xiong, J. & Zeng, Q. A new type of chiral cyclic sulfinamide–olefin ligands for rhodium-catalyzed asymmetric addition. Eur. J. Org. Chem. 32, 5360–5364 (2016).
Zhang, L., Tan, M., Zhou, L. & Zeng, Q. A novel, C2-symmetric, chiral bis-cyclosulfinamide-olefin tridentate ligand in Rh-catalyzed asymmetric 1,4-additions. Tetrahedron Lett. 59, 2778–2783 (2018).
Dobrydnev, A. V., Popova, M. V. & Volovenko, Y. M. Cyclic sulfinamides. Chem. Rec. 24, e202300221 (2024).
Liu, G., Cogan, D. A. & Ellman, J. A. Catalytic asymmetric synthesis of tert-butanesulfinamide. application to the asymmetric synthesis of amines. J. Am. Chem. Soc. 119, 9913–9914 (1997).
Han, Z. S. et al. Design and synthesis of chiral oxathiozinone scaffolds: efficient synthesis of hindered enantiopure sulfinamides and sulfinyl ketimines. Angew. Chem. Int. Ed. 52, 6713–6717 (2013).
Chen, Y., Wu, X., Yang, S. & Zhu, C. Asymmetric radical cyclization of alkenes by stereospecific homolytic substitution of sulfinamides. Angew. Chem. Int. Ed. 61, e202201027 (2022).
Han, Z. S. et al. Enantioselective synthesis of diverse sulfinamides and sulfinylferrocenes from phenylglycine-derived chiral sulfinyl transfer agent. J. Org. Chem. 76, 5480–5484 (2011).
Zhang, Y. et al. Asymmetric synthesis of sulfinamides using (−)-quinine as chiral auxiliary. J. Org. Chem. 77, 690–695 (2012).
Liu, Y., Wang, Z., Guo, B. & Cai, Q. Asymmetric synthesis of N-aryl sulfinamides: copper(I)-catalyzed coupling of sulfinamides with aryl iodides via kinetic resolution. Tetrahedron Lett. 57, 2379–2381 (2016).
Schreiber, S. L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287, 1964–1969 (2000).
Galloway, W. R., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80 (2010).
Zheng, W., Chen, X., Chen, F., He, Z. & Zeng, Q. Syntheses and transformations of sulfoximines. Chem. Rec. 21, 396–416 (2021).
Mock, W. L. & Nugent, R. M. Dipolar and concerted mechanisms in the diene reactions of N-sulfinylsulfonamides. J. Am. Chem. Soc. 97, 6526–6529 (1975).
Gais, H.-J., Scommoda, M. & Lenz, D. Rearrangement of allylic sulfoximines to allylic sulfinamides. Tetrahedron Lett. 35, 7361–7364 (1994).
Pyne, S. G., Dong, Z., Skelton, B. W. & White, A. H. Diastereoselective reductions of β-substituted-γ-keto sulfoximines and a novel palladium(0)-catalysed allylic sulfoximine to allylic sulfinamide rearrangement. J. Chem. Soc. Chem. Commun. 1995, 445–446 (1995).
Wang, G., Huang, H., Guo, W., Qian, C. & Sun, J. Unusual skeletal reorganization of oxetanes for the synthesis of 1,2-dihydroquinolines. Angew. Chem. Int. Ed. 59, 11245–11249 (2020).
Zeng, L. et al. Skeletal reorganization divergence of N-sulfonyl ynamides. Nat. Commun. 11, 5639 (2020).
Pyne, S. G., O’Meara, G. & David, D. M. Palladium(0) catalysed allylation reactions with racemic and enantiomerically pure allylic sulfoximines. Tetrahedron Lett. 38, 3623–3626 (1997).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines through the S-alkylation of sulfinamides. Angew. Chem. Int. Ed. 58, 17661–17665 (2019).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines via the S‑arylation of sulfinamides. J. Am. Chem. Soc. 141, 19263–19268 (2019).
Zhang, X., Ang, E. C. X., Yang, Z., Kee, C. W. & Tan, C.-H. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022).
Zhang, Y.-Q., Hu, L., Yuwen, L., Lu, G. & Zhang, Q.-W. Nickel-catalysed enantioselective hydrosulfenation of alkynes. Nat. Catal. 6, 487–494 (2023).
Huang, S. et al. Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds. Nat. Chem. 15, 185–193 (2023).
Tsuzuki, S. & Kano, T. Asymmetric synthesis of chiral sulfimides through the O-alkylation of enantioenriched sulfinamides and addition of carbon nucleophiles. Angew. Chem. Int. Ed. 62, e202300637 (2023).
Yang, G.-F. et al. Synthesis of chiral sulfonimidoyl chloride via desymmetrizing enantioselective hydrolysis. J. Am. Chem. Soc. 145, 5439–5446 (2023).
Zhang, Q., Xi, J., Ze, H. & Qingle, Z. Syntheses and transformations of sulfinamides. Synthesis 53, 2570–2582 (2021).
Robak, M. T., Herbage, M. A. & Ellman, J. A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 110, 3600–3740 (2010).
Yu, H., Li, Z. & Bolm, C. Copper-catalyzed transsulfinamidation of sulfinamides as a key step in the preparation of sulfonamides and sulfonimidamides. Angew. Chem. Int. Ed. 57, 15602–15605 (2018).
Fang, S., Liu, Z. & Wang, T. Design and application of peptide-mimic phosphonium salt catalysts in asymmetric synthesis. Angew. Chem. Int. Ed. 62, e202307258 (2023).
Wu, J.-H. et al. Towards axially chiral pyrazole-based phosphorus scaffolds by dipeptide-phosphonium salt catalysis. Angew. Chem. Int. Ed. 62, e202215720 (2023).
Pan, J. et al. Highly enantioselective synthesis of fused tri- and tetrasubstituted aziridines: aza-darzens reaction of cyclic imines with α-halogenated ketones catalyzed by bifunctional phosphonium salt. Angew. Chem. Int. Ed. 58, 7425–7430 (2019).
Zhang, H. et al. Regio- and stereoselective cascade of β, γ-unsaturated ketones by dipeptided phosphonium salt catalysis: stereospecific construction of dihydrofuro-fused [2,3-b] skeletons. Angew. Chem. Int. Ed. 60, 19860–19870 (2021).
Tan, J.-P. et al. Asymmetric synthesis of N-bridged [3.3.1] ring systems by phosphonium salt/Lewis acid relay catalysis. Nat. Commun. 13, 357 (2022).
Fang, S. et al. Enantiodivergent kinetic resolution of 1,1’-biaryl-2,2’-diols and amino alcohols by dipeptide-phosphonium salt catalysis inspired by the atherton-todd reaction. Angew. Chem. Int. Ed. 60, 14921–14930 (2021).
Dehli, J. R. & Gotor, V. Parallel kinetic resolution of racemic mixtures: a new strategy for the preparation of enantiopure compounds? Chem. Soc. Rev. 31, 365–370 (2002).
Wojaczyńska, E. & Wojaczyński, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Han, J. et al. Chiral sulfoxides: advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 47, 1307–1350 (2018).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian, Inc., 2013).
Acknowledgements
The authors sincerely thank Prof. Q.-H. Fan (Institute of Chemistry Chinese Academy of Sciences) for valuable discussions and suggestions. Financial support was provided by the National Natural Science Foundation of China (22222109, 21971165, 21921002 and 22371190), National Key R&D Program of China (2018YFA0903500), Beijing National Laboratory for Molecular Sciences (BNLMS202101), the Sichuan Science Foundation for Distinguished Young Scholars (2023NSFSC1921), Natural Science Foundation of Sichuan (2022NSFSC1181), Fundamental Research Funds from Sichuan University (2020SCUNL108) and Fundamental Research Funds for the Central Universities. We also acknowledge the comprehensive training platform of the Specialized Laboratory in the College of Chemistry at Sichuan University and the Analysis and Testing Center of Sichuan University for compound testing, particularly thank Dr. Jing Li and Dr. Dongyan Deng for NMR and HRMS testing.
Author information
Authors and Affiliations
Contributions
T.W. conceived and supervised the project. Z.L. and S.F. performed the experiments and analyzed the data. Z.L. carried out the synthesis of sulfoxide starting materials and collected the data. C.X. and K.X. gave suggestions and discussions on the mechanistic cycle. H.L. and Z.S. carried out the DFT calculations. Z.L. and S.F. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedicated to Professor Xiaoming Feng on the occasion of his 60th birthday.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Fang, S., Li, H. et al. Organocatalytic skeletal reorganization for enantioselective synthesis of S-stereogenic sulfinamides. Nat Commun 15, 4348 (2024). https://doi.org/10.1038/s41467-024-48727-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48727-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.