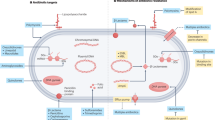
Abstract
Catheter-associated urinary tract infections (CAUTIs) are amongst the most common nosocomial infections worldwide and are difficult to treat partly due to development of multidrug-resistance from CAUTI-related pathogens. Importantly, CAUTI often leads to secondary bloodstream infections and death. A major challenge is to predict when patients will develop CAUTIs and which populations are at-risk for bloodstream infections. Catheter-induced inflammation promotes fibrinogen (Fg) and fibrin accumulation in the bladder which are exploited as a biofilm formation platform by CAUTI pathogens. Using our established mouse model of CAUTI, here we identified that host populations exhibiting either genetic or acquired fibrinolytic-deficiencies, inducing fibrin deposition in the catheterized bladder, are predisposed to severe CAUTI and septicemia by diverse uropathogens in mono- and poly-microbial infections. Furthermore, here we found that Enterococcus faecalis, a prevalent CAUTI pathogen, uses the secreted protease, SprE, to induce fibrin accumulation and create a niche ideal for growth, biofilm formation, and persistence during CAUTI.
Similar content being viewed by others
Introduction
Urinary catheterization is a common procedure to drain urine from patients’ bladders due to chronic conditions or while in healthcare facilities, intensive care units, during surgical procedures and recovery1,2,3. Despite its benefits, catheter placement increases the risk of developing a catheter-associated urinary tract infection (CAUTI)4,5,6,7. CAUTIs are one of the most common nosocomial infections and often lead to septicemia with a 30% mortality3,8. In fact, ~25% of sepsis cases come from complicated UTI, including CAUTIs9. Current CAUTI management focuses on catheter removal, replacement, and an antibiotic regimen6. However, the consistent colonization of catheters by external and fecal microflora, along with polymicrobial infections, pose challenges to management and treatment due to the increasing prevalence of antibiotic-resistant CAUTI pathogens6,10,11. Leading the CDC and WHO to classify CAUTI as a serious threat12,13.
Currently, there is no consensus on best practices for CAUTI treatment. Patients are treated with the same protocols as non-catheter associated (uncomplicated) urinary tract infections (uUTI). However, CAUTIs exhibit unique clinical manifestations, causative organisms, and pathologic mechanisms, making these infections distinct from uUTI6. For example, uUTIs are more prevalent in women than men (4:1 ratio) while in CAUTI there is no gender bias6. Also, E. coli accounts for >95% of uUTI, whereas CAUTI-pathogens are more diverse, including gram-negative, gram-positive, and fungal pathogens6.
In both humans and mice, urinary catheterization provokes local tissue damage to the bladder, activating coagulation, and converting the blood coagulation protein, fibrinogen (Fg), into fibrin. Fibrin clots and extravascular fibrin deposits promote blood clotting and wound healing14. Then, in fibrinolysis, plasminogen (Pg) is activated into serine protease plasmin to degrade fibrin clots and restore tissue homeostasis14,15. However, constant catheter-induced bladder inflammation induces Fg/fibrin accumulation on the catheter with increasing catheterization time in human and mice16,17. Fg/fibrin deposition compromises the urothelium, thereby introducing a platform for biofilm formation by CAUTI pathogens16,17,18,19,20,21,22,23.
Here, we identified that mice with genetic or acquired fibrinolytic-deficiencies are susceptible to severe and persistent CAUTI and systemic dissemination by diverse groups of prevalent uropathogens. Moreover, we found that E. faecalis, a prevalent CAUTI pathogen known to bind to Fg, secretes a protease, SprE, to induce fibrin accumulation and create a niche ideal for growth, biofilm formation and persistence during CAUTI. Furthermore, antifibrinolytic agent usage in catheterized mice results in Fg/fibrin accumulation in the bladders, resulting in persistent CAUTI and systemic dissemination in mono- and poly-microbial infection by E. faecalis, E. coli, and C. albicans. Identification of patient populations with higher susceptibility to CAUTI and its sequelae will allow clinicians to improve patient outcomes by implementing efficient patient monitoring to mitigate infection incidences, morbidities, and mortality.
Results
Prolonged urinary catheterization promotes enterococcal burden and persistence in the bladder and systemic dissemination
Clinical studies showed that CAUTI-burdened patients have an increased risk of septicemia and mortality10,11,24,25,26. To understand E. faecalis bladder and catheter colonization progression and systemic dissemination, we performed a temporal study examining acute infection (1, 3, 6, 9, 12 hr post-catheterization and infection (hpi) and 1 day post-infection (dpi)) and prolonged infection (3, 7, 14 dpi). Female C57BL/6 mice were catheterized and infected with of E. faecalis (~2 ×107 CFU) or mock-infected (PBS). At specified timepoints, mice were sacrificed to harvest the bladders, catheters, kidneys, spleens, and hearts. Bladders at 1 hpi have an initial colonization of ~104 bacterial CFU that increased significantly overtime, showing maximum colonization at ~107 CFU by 12 hpi in the bladder and persisting through 14 dpi (Fig. 1a). Similarly, catheter colonization significantly increased with catheterization time and persisted (Fig. 1b). Importantly, robust bladder and catheter colonization allowed E. faecalis to disseminate to the kidneys, spleen, and heart (Fig. 1c–e). Thus, our data recapitulates what is clinically observed11,12,25,26,27.
a–e E. faecalis establishes persistent colonization overtime. Mice were catheterized and infected with 2 × 107 CFU of E. faecalis OG1RF. Enterococcal colonization of organs and catheters were assessed by quantifying bacterial burden. f Bladder weights of naïve (non-implanted control) mice and implanted mice in the presence or absence of the E. faecalis at the indicated times. g Bladder sections were stained with H&E to compare inflammation from catheterization in the presence or absence of the pathogen overtime (scale bars: 500 µm). h Heatmap represents catheterized bladder cytokines fold changes over naïve bladder in the presence or absence of the E. faecalis OG1RF infection at the indicated times. i IL-6 analysis levels in the catheterized bladder (with or without infection), bloodstream, and liver. The horizontal broken line represents twofold change cytokine levels compared with naïve control mice over a 2-fold change with the bar representing the mean ± SD. j Comparison of the IL-1α (octagons), IL-1β (diamonds), IL-6 (circle), and TNFα (square) levels in urine from patients catheterized for 24 h and healthy donors (NC; non-catheterized). Two-tailed Mann–Whitney U test was used to determine significance; *P < 0.05 was considered statistically significant. **P < 0.005; ***P < 0.0005; ****P < 0.0001. The horizontal bar represents the median value with range as error bars. The horizontal broken line represents the limit of detection of viable bacteria. LOD limit of detection. Infections were done in three independent experiments with n = 6 mice for each one, and data are shown as the bacterial CFU/organ or catheter. Animals that lost the catheter were not included in this work. All n values, exact p-values, and source data are provided in Source Data file.
Progression of bladder inflammation correlates with microbial burden
Catheter-induced inflammation is a CAUTI hallmark21,27,28. To understand how urinary catheterization changes the bladder environment during acute and prolonged catheterization and in the presence or absence of infection; we examined mouse bladder edema and inflammation by bladder weight and histological analysis. Bladders exhibited a progressive weight increase with the catheter dwell time (Fig. 1f). Bladder histological analysis corroborates the gradual edema progression and increase of the bladder size associated with urinary catheterization (Fig. 1g). Furthermore, there is a significant positive correlation of bladder weight with bacterial burden in the bladder (r = 0.7991; P = 0.0098) or catheter (r = 0.9005; P = 0.0008) (Supplementary Fig. 1). Importantly, E. faecalis infection further increased bladder weight after 12 hpi (Fig. 1f). This indicates that catheterization alone increases bladder edema overtime and is exacerbated by an E. faecalis infection.
E. faecalis presence modulates levels of inflammatory cytokines in the catheterized bladder
Previous studies show that acute urinary catheterization induces many inflammatory cytokines in the bladder27, of which IL-1β, IL-6, 12(p40), IL-17, CSF 3, and CXCL1 are further induced during E. faecalis infection28. Since E. faecalis significantly exacerbates bladder inflammation during catheterization (Fig. 1f, g), we profiled cytokine level changes of E. faecalis- or mock-infected catheterized bladder, relative to naïve bladders. We found that E. faecalis infection altered cytokine expression patterns differently than in mock-infected bladders. IL-6, CSF 3, CXCL1, and IL-17 levels were significantly higher during infection (Fig. 1h, i, and Supplementary Fig. 2). Conversely, infection significantly reduced levels of IL-1α, IL-2, IL-9, IL-10, IL-12 (p70), IL-13, IFNγ, IL-3, CCL4 (MIP-1β), CSF 2 (GM-CSF), CCL5 (RANTES), and TNFα (Fig. 1h, Supplementary Figs. 2 and 3a, c). IL-1β, IL-12 (p40), IL-4, and CCL3 levels were modulated, showing significantly lower induction during early infection (1–9 h) followed by a significant increased after 12 h (Fig. 1h, Supplementary Figs. 2 and 3b). IL-5 and CCL2 levels increased regardless of the infection (Fig. 1h and Supplementary Fig. 2). This indicates that there is a differential inflammatory cytokine profile on infected catheterized bladders.
During catheter-induced bladder inflammation in mice and humans16,17, Fg, a liver-produced proinflammatory protein, is recruited into the bladder and deposited on urinary catheters, serving as a platform for microbial CAUTI16,18,21,22,23,24,29. IL-1 (α and β), IL-6, and TNFα are known to stimulate Fg production by migrating from damaged tissues, through the bloodstream and signaling to Fg-producing hepatocytes in the liver30,31. To understand their contribution to CAUTI-associated inflammation, we evaluated levels of these proinflammatory mediators in the bladder. High levels of IL-6 during catheterization were observed, which significantly increased during infection (Fig. 1h, i). We also assessed the levels of all four cytokines in the bloodstream and livers. Expectedly, IL-6 levels were elevated in the bloodstream and liver (Fig. 1i and Supplementary Fig. 3). To understand the role of IL-1α, IL-1β, IL-6 and TNFα in human urinary catheterization, we collected urine from patients that had a urinary catheter for ~24 h and from healthy donors to compare and analyze their levels. We found that IL-1α, IL-1β, IL-6 and TNFα levels significantly increased in urine from catheterized patients when compared with urine from non-catheterized healthy donors (Fig. 1j). Furthermore, IL-6 was significantly elevated compared with IL-1α, IL-1β, and TNFα in the catheterized patients (Fig. 1j). A limitation of this study is that urine samples were not collected from catheterized patients before catheterization as a further pair-wise comparison. Without this data, any comorbidity prior to catheterization could also contribute to the cytokines present in patient urine post-catheterization. However, based on this data provided as is, we can speculate IL-6 could be an important cytokine during human and mouse CAUTIs and, in mice, IL-6 may communicate with the liver’s Fg expression and release, resulting in its accumulation in the catheterized bladder. Further work should be done to fully assess this.
Fg deposition increases on urinary catheters during E. faecalis infection
Previously, clinical and animal studies from our and other groups have shown that Fg is a major component of proteins deposited on urinary catheters16,17,19,20,21,23,32,33. Importantly, our previous immunofluorescence analyses on catheters showed increased Fg deposition during E. faecalis infection16,17,18, suggesting that infection may promote Fg recruitment. To test this, we performed a temporal quantitative proteomic analysis to compare Fg abundance on urinary catheters by analyzing the three Fg chains, α, β, γ, between catheterized (mock-infected) and catheterized and infected mice. Five catheters were harvested at the indicated timepoints, pooled, processed, and trypsin digested. Total protein analysis showed 230 proteins associated with mouse catheters (Fig. 2a, c, and Supplementary Data 1). Fg is among the most abundant deposited proteins on the catheter, corroborating our previous proteomic study at 24 hpi19 and its abundance significantly increased in acute and prolonged infection starting 1 dpi (Fig. 2b, Supplementary Fig. 4, and Supplementary Data 1). Higher Fg -α, -β, -γ total peptide counts in infected bladders suggest that E. faecalis may have a mechanism to promote Fg accumulation.
a Venn diagram comparing deposited proteins on urinary catheters retrieved from patients and mice catheterized with a dwell time of 1 day using E Venn online software81. Red rectangles represent inflammatory and immune proteins, green circles represent coagulation cascade proteins, purple ovals represent complement cascade proteins, while black dots represent other proteins. b Fg levels in acute and prolonged catheterized bladder in the absence or presence of E. faecalis OG1RF infection. c Metascape analysis including a hypergeometric test and Benjamini–Hochberg p-value correction showing the top significant pathways shared proteins found on mice and human catheters based on corrected -log(p-value). Metascape network analysis and clusters of interactions of the top significant pathways were visualized with Cytoscape82. Colors on bar graph correspond to the gene ontologies on the adjacent network analysis. Source data is provided in Source Data file.
Coagulation and immune response pathway proteins are found on human and mouse urinary catheters
To understand if similar host proteins are deposited in human urinary catheter, we performed a proteomic analysis of 10 human urinary catheters with a 24–26 h dwell time, finding a total of 104 host deposited proteins, including several coagulation factors, including Fg, alpha-1 antitrypsin (A1AT), alpha-2-macroglobulin (A2M), plasminogen (Pg), antithrombin III (AT3), thrombin (THB), and kininogen (KNG) (Fig. 2a, Supplementary Data 2). Resembling mouse catheters, Fg is one of the most abundantly deposited protein. Comparative analyses showed 76 (73% of the identified human proteins) shared deposited proteins between mouse and human catheters (Fig. 2a). The 76 shared proteins were subjected to an unbiased and comprehensive pathway enrichment analyses using Metascape34, finding that the top significant pathways enriched are part of host inflammation, immune, hemostasis, and wound healing responses, including platelet degranulation, neutrophil degranulation, complement and coagulation cascades (Fig. 2a, c, Supplementary Fig. 5a, and Supplementary Table 1). Furthermore, we found that transcriptional regulators involved in inflammation and immune responses, including CEBPA, STAT3, and NFKB1, were highly predicted in the catheterized bladder environment (Supplementary Fig. 5b and Supplementary Table 2). Additionally, we performed network analysis of differential proteins found ether in mouse or patient catheters (Supplementary Fig. 6), finding that some of the proteins are also involved in immune and hemostasis processes described in the shared proteins (Fig. 2). Collectively, proteomics and ontology relationships indicate that coagulation and wound healing pathways are active in mouse and human catheterized bladders.
Fibrinogen is critical for colonization of the catheterized bladder and catheter
To investigate if Fg is critical for enterococcal CAUTI establishment, we compared bacterial colonization in C57BL/6 wild-type (WT) mice to several mutant mice (C57BL/6-background), including: i) Fg deficient mice (Fg−/−)35; ii) mice expressing a mutant form of Fg that cannot be converted to fibrin by thrombin, remaining a monomer (FgAEK)36; and iii) FVIItTA/tTA (FVIItTA)37 mice, which are hypomorphic for coagulation factor VII (FVII)38, resulting in severe thrombin production downregulation, reducing fibrin formation (Fig. 3a). Mice were catheterized and infected; then at 1 dpi, bacterial burden was assessed in organs and catheters. Compared to WT mice, Fg−/− mice exhibited significantly reduced enterococcal burdens in bladders (~1.5 logs) and catheters (~3.5 logs) (Fig. 3b, c). Similarly, FgAEK mice exhibited significant defects in bladder and catheter colonization (Fig. 3b, c). Fibrin reduction in the bladder via FVIItTA, resulted in significantly lower colonization in the bladders (~1 log) and catheters (~1.5 logs) (Fig. 3b, c). Soluble Fg presence (FgAEK) significantly decreased kidney dissemination (Fig. 3d). Enterococcal dissemination to the spleen and hearts was not observed in any of the mouse lines (Fig. 3e, f). These results further confirm that Fg is critical for enterococcal CAUTI. Importantly, soluble Fg or low fibrin formation also results in lower colonization, indicating that fibrin accumulation is critical for persistent colonization.
a Coagulation cascade diagram (color boxes correlates with mouse strains used in this study). C57BL/6 wild type (WT) mice and transgenic coagulation mutants in C57BL/6-background looking at clot formation pathway (b–f) or fibrinolytic system (g–k) were catheterized and infected with ~2 × 107 CFU of E. faecalis OG1RF. After 24 hpi, bacterial burdens were measured in bladder tissues (a, g), catheters (b, h), kidneys (c, i), spleen (d, j), and hearts (f, k). For b–f: n = 22 for +/+, n = 8 for Fg−/−, n = 10 for FVIItTA, and n = 9 for FgAEK. For g–k) n = 19 for +/+, n = 9 for Pg−/−, n = 9 for PGB, n = 8 for uPA−/−, n = 6 for tPA−/−, and n = 6 for PAI−/−. The Kruskal–Wallis test followed by a two-tailed Dunn’s test was used to determine significance; *P < 0.05 was considered statistically significant. **P < 0.005; ***P < 0.0005; ****P < 0.0001. The horizontal bar represents the median value. The horizontal broken line represents the limit of detection of viable bacteria. LOD limit of detection. Colors are used to help distinguish between genotypes. For CFU enumeration, infections were done in at least three independent experiments with n = 3–6 mice depending on the genotype, and data are shown as the bacterial CFU/organ or catheter. Total n Animals that lost the catheter were not included in this work. All n values, exact p-values, and source data are provided in Source Data file.
Fibrin accumulation enhances enterococcal bladder and catheter persistence during CAUTI
Fibrinolysis is critical for dissolving fibrin net/clots and defective fibrinolysis results in fibrin accumulation. Based on this and that Pg was found on the human and mouse catheters (Fig. 2a), we investigated fibrinolysis in CAUTI, focusing in Pg and its key activators. We used C57BL/6-background mice deficient in different fibrinolysis factors (Fig. 3a). To increase fibrin accumulation, we used mouse lines deficient for: i) plasminogen (Pg−/−)39, ii) urokinase plasminogen activator (uPA−/−)40, and iii) tissue plasminogen activator (tPA−/−)40. To decrease fibrin levels, we used a deletion of plasminogen activator inhibitor (PAI−/−)41, which exhibits unregulated fibrin degradation (Fig. 3a).
We found that persistent fibrin accumulation led to significant increases of enterococcal bladder (~2 logs) and catheter (~1.5 logs) colonization in Pg−/− mice when compared to WT mice (Fig. 3g, h). Importantly, fibrin reduction in the bladder via PAI deficiency, resulted in significantly lower bladder (~1 log) and catheter (~1.5 logs) colonization (Fig. 3g, h).
To further confirm that inactivation of Pg proteolytic activity correlates with enterococcal colonization enhancement, we used a transgenic mouse expressing Pg with a plasmin-inactivating active site mutation (PGB)42, finding that bacterial burden in the bladder and catheter was similar to Pg−/− mice (Fig. 3g, h). This suggests that fibrinolysis inhibition correlates with exacerbating E. faecalis colonization.
Importantly, bladder and catheter bacterial colonization in uPA−/− mice significantly increased similarly to Pg−/− mice, whereas tPA−/− mice exhibit similar bacterial burden to WT mice. Thus, uPA is the primary activator of Pg into plasmin in the catheterized bladder (Fig. 3g, h). Since coagulation dysregulation is not uncommon in human populations (Supplementary Table 3), these data suggest that coagulation deregulation could have a direct impact on enterococcal CAUTI.
Host fibrinolytic deficiencies predispose the host to enterococcal systemic dissemination
We further assessed the effect of Fg/fibrin modulation in enterococcal dissemination, finding that fibrinolytic defects (Pg−/−, PGB, and uPA−/−) significantly increased bacterial burden in kidneys, spleen, and hearts compared with WT mice colonization (Fig. 3i–k). Furthermore, tPA deficiency resulted in a bimodal kidney enterococcal colonization (Fig. 3i). Interestingly, kidneys with higher enterococcal colonization came from mice with higher bladder colonization, suggesting that a persistent bladder colonization may result in their dissemination (Fig. 3i). These demonstrate that fibrin accumulation predisposes the host to enterococcal systemic dissemination.
E. faecalis proteases degrade plasmin and plasminogen but not thrombin
We previously demonstrated that during CAUTI and growth in urine conditions, E. faecalis OG1RF induced the expression and activity of two enterococcal secreted proteases, a serine protease (SprE) and metalloproteinase gelatinase (GelE), which are critical for enterococcal CAUTI and systemic dissemination29. Given that E. faecalis in the catheterized bladder increases Fg/fibrin levels (Fig. 2b) and that fibrinolysis deficiencies enhance enterococcal colonization (Fig. 3), we examined if the enterococcal secreted proteases antagonizes the fibrinolytic system by assessing their ability to degrade human Pg (hPg) and plasmin (hPm) in vitro.
We compared the protease activity of WT, mutants defective for each protease (ΔsprE or ΔgelE), or both proteases (ΔgelEΔsprE) against hPg and hPm under urine conditions (See Methods, Supplementary Fig. 7). Filtered WT supernatants degraded hPg (~95 kDa) and hPm (~85 kDa), producing a major degradation fragment ~35 kDa and 3 visible degradation products (DP) between 50–40 kDa (Fig. 4a, b). WT and ΔgelE supernatants produced similar hPg and hPm degradation pattern while the ΔsprE supernatant was not able to fully degrade hPg and hPm, showing three faint fragments between 50–40 kDa and no major degradation product at ~35 kDa (Fig. 4a, b). The ΔgelEΔsprE mutant completely lost the ability to degrade hPg and hPm into smaller peptides, similar to urine or PBS controls (Fig. 4a, b, d, e).
SDS-PAGE analysis of the proteolytic activity of E. faecalis WT and protease mutants’ cell-free supernatants against purified (a) plasminogen, (b) plasmin, or (c) thrombin and their corresponding degradation quantification by densitometry (d–f). g 24 h catheterized PGB bladder homogenates were incubated with E. faecalis WT and protease mutants’ cell-free supernatants and proteolytic activity against plasminogen and thrombin was monitored by Western blots. h, i Densitometry analysis of the Pg and thrombin degradation by bacterial supernatants performed in g. j–m SprE degradation of plasmin results in inhibition of fibrinolysis. To test this, supernatants E. faecalis grown in urine were filtered and concentrated, then incubated plasmin was for 4 h at 37 °C; then each mixture was incubated with purified (j) fibrin or (k) Pg−/− mouse bladder homogenates from 24 h catheterized non-infected mice. Degradation of fibrin was monitored by detection of Fragment E in incubation with fibrin or bladder homogenates by SDS-PAGE or western blot analysis, respectively (j, k). Fragment E quantification by densitometry obtained in l and m. CRM, cross reactive material. β-actin was used as loading and normalization control. An ANOVA followed by a two-tailed Tukey’s post-hoc was used to determine significance; *P < 0.05 was considered statistically significant. **P < 0.005; ***P < 0.0005. For d–f: n = 5; h, n = 8; i, n = 5; and l, m, n = 5. The horizontal bar represents the median value. Blot images (g, k) were processed in parallel and sample processing controls were run on different gels. All n values, exact p-values, and source data including uncropped gel images are provided in Source Data file.
To understand whether these proteases are promiscuous and capable of targeting other factors in the coagulation cascade, specifically the fibrin formation pathway, we incubated the strains’ supernatants with thrombin, finding that thrombin was not degraded in any of the treatments (Fig. 4c, f). To further explore SprE specificity, we tested degradation of other host proteins with comparable size to Pg and Pm, prekallikrein (~90 kDa), factor XII (FXII; ~80 kDa), and endoplasmin (~110 kDa). Prekallikrein and FXII are part of the coagulation cascade (Fig. 3a) and endoplasmin (HSP090b1; ~110 kDa observed) was found deposited on both mouse and patient catheters with an expected size of ~90 kDa, similar to Pg (Supplementary Data 1 and 2). We found differential degradation of these proteins by the enterococcal proteases (Supplementary Fig. 8), GelE was important for degradation of FXII while SprE was important for degradation for prekallikrein but dispensable for endoplasmin and FXII. This suggests that enterococcal secreted proteases could modulate the coagulation cascade and other host pathways.
To further validate the Pg and Pm degradation, we determined the proteolytic activity of supernatants from E. faecalis WT and various mutants against mouse Pg and thrombin in a complex catheterized environment. Cell-free bacterial supernatants were incubated with bladder homogenates (1:5) from PGB mice catheterized for 24 h (Supplementary Fig. 7). WT and ΔgelE supernatants significantly degraded Pg while no degradation was observed in the urine control, the ΔsprE single mutant and the double ΔgelE ΔsprE mutant supernatant (Fig. 4g, h). No cleavage of thrombin was observed with any of the treatments (Fig. 4g, i).
To determine whether E. faecalis proteases could functionally disrupt fibrinolysis by targeting plasmin, we incubated purified plasmin with WT and mutant strains’ supernatants, followed by a secondary incubation with thrombin-generated fibrin (Fig. 4j) or Pg−/− homogenates (Fig. 4k) that were catheterized, non-infected, for 24 h (Supplementary Fig. 7). Supernatants-containing SprE (WT and ΔgelE) degraded plasmin, resulting in lack of fragment E generation, demonstrating plasmin inhibition (Fig. 4j–m). These data suggests that E. faecalis’ SprE protease activity in urine targets the fibrinolytic system by inactivating Pg and plasmin, which results in dysregulated fibrin accumulation.
Fibrin accumulation rescues E. faecalis ΔgelEΔsprE colonization deficiency
If enterococcal protease actively modulates Fg and fibrin levels to enhance CAUTI colonization, then the colonization defect observed in the absence of protease activity should be reversed in mutant mice that accumulate fibrin. Expectedly, defective colonization of E. faecalis ΔgelEΔsprE was further reduced in Fg−/− mice. Predictively, defective colonization was rescued in mice that accumulate fibrin due to fibrinolytic deficiencies (Pg−/− and PGB) (Fig. 5a, b), exhibiting higher than the WT strain colonization. Importantly, fibrin accumulation in the catheterized bladder promoted E. faecalis ΔgelEΔsprE systemic dissemination (Fig. 5c–e). This further demonstrates E. faecalis’ ability to modulate the coagulation cascade, creating an environment conducive to its colonization.
a–e C57BL/6 WT and coagulation deficient mice were infected with ~2×107 CFU of E. faecalis OG1RFΔgelEΔsprE or (f–j) uropathogenic E. coli UTI89. After 24 hpi, bacterial burdens were measured in bladder tissues (a, f), catheters (b, g), kidneys (c, h), spleen (d, i), and hearts (e, j). The Kruskal–Wallis test followed by a two-tailed Dunn’s test was used to determine significance; *P < 0.05 was considered statistically significant. **P < 0.005; ***P < 0.0005; ****P < 0.0001. The horizontal bar represents the median value. The horizontal broken line represents the limit of detection of viable bacteria. LOD limit of detection. For CFU enumeration, infections were done at least in 3 independent experiments with n = 3–6 mice depending on the mouse genotype, and data are shown as the bacterial CFU/organ or catheter. Total n counts for a–e: are n = 17 for WT mice with WT E. faecalis; n = 18 for WT mice and double mutant E. faecalis; n = 6 for Fg−/−; n = 7 for Pg−/−; and n = 9 for PGB. Total n counts for f–j: are n = 13 for WT; n = 6 for Fg−/−; n = 6 for FgAEK; n = 6 for PG−/−; and n = 6 for PGB. Animals that lost the catheter were not included in this work. All n values, exact p-values, and source data are provided in Source Data file.
Hosts with fibrinolytic deficiencies enhance uropathogen E. coli UTI89 CAUTI colonization and systemic dissemination
Recently, we found that using a novel liquid-infused silicone catheter material to reduce Fg deposition on urinary catheters resulted in decreased bladder and catheter colonization and systemic dissemination by uropathogenic E. coli UTI89 in our mouse CAUTI model19. Here, we found that bladder and catheter colonization in Fg−/− mice or coagulation-deficient mice (FgAEK; soluble Fg) was significantly reduced (Fig. 5f, g). Conversely, not only was UTI89 bladder and catheter colonization significantly increased (~2 logs) in mice with fibrinolytic deficiencies (Pg−/− and PGB) (Fig. 5f, g), but these mice also experienced significant systemic dissemination (Fig. 5h–j). These data provide evidence that uropathogenic E. coli colonization is modulated by Fg/fibrin in the catheterized bladder.
Antifibrinolytic agent promotes a persistent CAUTI and systemic dissemination by diverse uropathogens
Antifibrinolytic agents including tranexamic acid (TXA) are often used in patients with bleeding disorders or patients undergoing surgical procedures43,44 (Supplementary Table 3). TXA is extensively used to treat patients with postpartum hemorrhages, traumatic injury, and surgical procedures that increase the risk of bleeding44,45. TXA is a synthetic anti-fibrinolytic amino acid that acts by competitively blocking the lysine binding sites on plasmin(ogen), inhibiting plasmin interaction with fibrin45 (Fig. 6a). As 86% of surgery patients require urinary catheterization46, we assessed whether TXA treatment during urinary catheterization increased fibrin accumulation in the bladder, enhancing colonization and systemic dissemination by three of the most prevalent CAUTI uropathogens: E. faecalis, E. coli, and the fungal pathogen C. albicans6,47. Mice were treated with TXA or vehicle control (PBS) intraperitoneally (Fig. 6b). Then, mice were catheterized and infected with the respective pathogen and sacrificed at 1 dpi. TXA-treated mice exhibited a significant increase of bladder and catheter colonization and promoted systemic dissemination of all pathogens when compared with PBS-treated mice (Fig. 6c–e). No significant differences in bladder weights between treatments were observed, suggesting that TXA did not exacerbate catheter-induced inflammation (Fig. 6f). To confirm fibrinolysis inhibition and fibrin accumulation, Fg/fibrin concentration and fragment E generation was compared and quantified between PBS- and TXA-treated bladders (Fig. 6g–i) and further confirmed in vitro (Fig. 6j). We found that Fg/fibrin concentration increased and fragment E generation decreased in TXA-treated bladders (Fig. 6g–i). Since Fg induces IL-6 expression in immune and epithelial cells48,49, we measured IL-6 levels in the bladder. We found that IL-6 levels significantly increased in TXA-treated mice (Fig. 6k). This is consistent with increased Fg/fibrin levels in the TXA-treated bladders, suggesting a positive-feedback loop between the inflamed bladder and Fg in the catheterized host.
a Tranexamic acid (TXA) targets plasmin, thus inhibiting the fibrinolytic cascade. b Treatment timeline. C57BL/6 WT mice were infected with ~2 × 107 CFU of E. faecalis OG1RF (c),~2 × 107 CFU of E. coli UTI89 (d), or ~1 × 106 CFU of C. albicans SC5314 (e) and dosed with either TXA (100 mg/mL i.p.) or vehicle (PBS). After 24 hpi, pathogen burdens were enumerated in bladder tissues, catheters, kidney pairs, spleens, and hearts. f Bladder edema was assessed after tissue harvest by weighing bladders. g–i Bladder homogenates were diluted (1:10) and analyzed for (g) fibrinogen, (h) fibrin degradation, and (k) IL-6. Fibrinogen and IL-6 levels were analyzed via ELISA. Fibrin degradation in the bladder homogenates was analyzed by measuring fragment E production via western blot and β-actin was used as loading and normalization control (h) and quantified by densitometry (i). TXA inhibition of plasmin-dependent fibrin degradation was further confirmed and analyzed in vitro using purified human proteins via western blot (j). l–o Polymicrobial CAUTI with E. faecalis, E. coli, and C. albicans. l Bladder edema of mice treated with TXA or vehicle during polymicrobial CAUTI. m–o C57BL/6 WT mice were infected with ~2 × 107 CFU of E. faecalis OG1RF (m),~2×107 CFU of E. coli UTI89 (n), and ~1 × 106 CFU of C. albicans SC5314 (o) and dosed with either TXA (100 mg/mL i.p.) or vehicle (PBS). Pathogen burden was assessed after 24 hpi in bladder tissues, catheters, kidney pairs, spleens, and hearts. The Mann–Whitney U test was used to determine significance; *P < 0.05 was considered statistically significant. **P < 0.005; ***P < 0.0005; ****P < 0.0001. The horizontal bar represents the median value. The horizontal broken line represents the limit of detection of viable pathogen. LOD limit of detection. For CFU enumeration, infections were done at least in 3 independent experiments with n = 3–6 mice each one, and data are shown as the microbial CFU/organ or catheter. For c: n = 14 for vehicle and n = 15 for TXA. For d: n = 10 for vehicle and n = 9 for TXA. For e: n = 19 for vehicle and n = 16 for TXA-treated. For f: n = 10 for vehicle + E. faecalis, n = 8 for TXA + E. faecalis, n = 10 for vehicle + E. coli, n = 9 for TXA + E. coli, n = 8 for vehicle + C. albicans, and n = 7 for TXA + C. albicans. For i–k: n = 6 for all vehicle and n = 9 for all TXA. For l–o: n = 11 for all vehicle and n = 12 for all TXA-treated. Animals that lost the catheter were not included in this work. All n values, exact p-values, and source data are provided in Source Data file.
We further tested whether fibrin accumulation could enhance polymicrobial CAUTI. For this, TXA and PBS-treated mice were catheterized and infected with all three uropathogens, E. faecalis, E. coli, and C. albicans6,47. We found no difference in inflammation between PBS- and TXA-treated mice (Fig. 6l). However, blocking fibrinolysis with TXA significantly enhanced the burden of all pathogens in the bladders and catheters and increased systemic dissemination (Fig. 6m–o). In conclusion, these data show that pharmacological inhibition of fibrinolysis in the host enhances mono- and polymicrobial CAUTI and systemic dissemination by diverse uropathogens.
Discussion
CAUTIs remain prevalent and costly, increasing patient morbidity and mortality50,51. CAUTI prevention and treatment faces major challenges, including the ability to predict: i) when catheterized patients develop infections; ii) which uropathogen will cause the infection; and iii) which patient population is more susceptible to septicemia. Previous attempts at treating CAUTIs include vaccine development, antibody therapy, and compounds that target specific pathogens6,52. However, multiple pathogens from different kingdoms can simultaneously cause CAUTIs and the risk of developing an infection increases with dwelling time. Efficiently predicting when patients will develop an infection, the causative organism, and which patient populations are more susceptible to systemic dissemination is imperative for successful CAUTIs prevention and treatment. Thus, a better understanding of CAUTI pathophysiology is critical to develop better clinical practices for improving patient outcomes.
Here, we found that the coagulation cascade plays an important role in CAUTI outcome. Importantly, we identified that host coagulopathies that result in fibrin clot accumulation promote higher pathogen colonization in the catheterized bladder by the most prevalent uropathogens in mono- or poly-microbial infections, enhancing persistence during CAUTI and promoting bloodstream infection and systemic dissemination (Figs. 3, 5, and 6). Fibrin is a major thrombus-formation end product53 and its concentration is tightly controlled by a series of cofactors, inhibitors, and receptors15. Once healing is resolved, the fibrinolytic pathway is triggered by activation of Pg into plasmin by uPA and tPA, promoting fibrin clot degradation and restoring tissue homeostasis (Fig. 3a)14,15. In the catheterized bladder, our results show that uPA, which was originally isolated from human urine, is the main Pg activator, but not tPA (Fig. 3). This could be related to their action mechanisms as tPA activation of Pg requires attachment to the fibrin on the clot surface to activate fibrin-bound Pg54. Since CAUTI pathogens form biofilms on fibrin, they may occlude tPA binding. In contrast, uPA is fibrin-independent and activates Pg in solution or when associated with its cellular receptor uPAR55, providing a cell-mediated Pg activation56. uPAR is present on many immune cells including macrophages56 that are recruited by urinary catheterization20,27,28; thus, it is possible that these immune cells are important for uPA-cell-mediated Pg activation. The uPA activation mechanism of Pg will require further experimentation.
Hypofibrinolysis and several thromboembolic diseases, including strokes, venous thromboembolism, rheumatoid arthritis, and renal diseases are accompanied by fibrin accumulation57,58. Furthermore, there are several congenital deficiencies that result in fibrin clot accumulation, including individuals with deficiencies of Pg, tPA, uPA, PAI-1, PAI-2, TAFI, and Annexin A253 (Supplementary Table 3). Consistent with our CAUTI data, fibrin accumulation from imbalance between activation of coagulation and inhibition of fibrinolysis, is detrimental for patients suffering from COVID-1959 and ventilator-associated pneumonia60. Moreover, fibrin accumulation can also result from antifibrinolytic treatments, which are commonly use in patients with bleeding disorders58,61 (Supplementary Table 3), in patients with traumatic injuries, or undergoing surgical procedures that increase bleeding risk, including liver transplants, cardiac and cesarean surgeries43,44,45,62. TXA is an active inhibitor of uPA and tPA, and also plasmin-fibrin binding63,64. Importantly, TXA elimination from the body is by urinary excretion, increasing its concentration in urine62,63. This information further validates our results showing that TXA is an efficient antifibrinolytic agent in the catheterized bladder, resulting in fibrin accumulation and enhancing CAUTI and higher systemic dissemination. Based on our data, these patient populations with coagulopathies or treatments that result in fibrin accumulation, if receiving a urinary catheter, may have a higher risk of developing a CAUTI and could be more susceptible to secondary bacterial and fungal infection from a CAUTI.
During catheterization, IL-6 is the most highly elevated cytokine in the bladder, bloodstream, and liver and E. faecalis infection promotes its concentration (Fig. 1i). IL-6 is a potent regulator of inflammation, coagulation, and the immune response65 by regulating neutrophil infiltration, monocytes to macrophages differentiation, and Fg expression by hepatocytes66,67. Interestingly, Fg/fibrin also stimulates IL-6 expression by immune cells in the damaged tissue56,68; thus, resulting in a positive-feedback loop, dysregulated inflammation, and Fg/fibrin accumulation, promoting microbial colonization and perpetuating a futile inflammation cycle that benefits the pathogen. Importantly, IL-6/Fg dysregulation play a role in chronic inflammatory diseases and cancer30,31,66. IL-6 pathway activation was further supported by Metascape analysis showing that CEBPA and STAT3 are highly predicted transcriptional regulators of the catheterized bladder environment (Supplementary Fig. 5b). IL-6 levels has been associated with severe and febrile uUTI69. However, during early, recurrent, or asymptomatic uUTI, IL-6 is repressed, unchanged or low levels70,71,72,73,74. In fact, TNFα is elevated during acute uUTI and is associated with recurrent uUTI73,74. Importantly, TNFα is repressed during CAUTI (Fig. 1h, Supplementary Fig. 3). This further demonstrates that uUTI and CAUTI have different pathophysiologies. Therefore, targeting IL-6/Fg signaling to modulate its effects on pathogenesis may provide a novel approach to improving CAUTI outcomes.
Here, we found that SprE actively degrades Pg and plasmin, suggesting that E. faecalis uses SprE to inactivate the fibrinolytic system. This strategy differs from other bacterial pathogens, including Group A Streptococcus (GAS), which activates Pg into plasmin via streptokinase, dissolving blood clot to cause an invasive infection75. Importantly, SprE specificity against Pg, Pm, and prekallikrein, suggests that SprE is recognizing a specific recognition site. Therefore, identifying Pg/plasmin or prekallikrein cleavage sites using purified SprE will be important to determine its action mechanism. Furthermore, it would be interesting to understand if other uropathogens produce secreted proteases with similar SprE activity to target the fibrinolytic system to enhance their colonization.
E. faecalis induction of fibrin accumulation creates ideal conditions for biofilm formation and persistence in the bladder, which is an open and dynamic system, where urine is constantly passing. Fibrin accumulation also contribute to the Fg/IL-6 feedback loop. This further explains how E. faecalis can thrive and exploit the catheterized bladder despite robust inflammatory responses and how fibrin accumulation is also conducive for CAUTI and systemic dissemination by other pathogens. Enterococci’s ability to modulate the bladder environment to benefit itself and other pathogens may further explain why enterococci are described as a pioneer species on urinary catheter23. This supports several clinical studies that showed enterococci are early colonizers and persist during prolonged catheterization in monomicrobial or polymicrobial interactions5,23,76.
In uUTI, E. coli uses the type 1 pilus to bind mannosylated-receptors on the urothelial surface to invade and form intracellular bacterial communities (IBCs)52. During E. coli CAUTI, IBC formation is significantly reduced without affecting overall bladder colonization77,78. We found that Fg, which is highly glycosylated, is used by E. coli colonize the bladder and catheter during CAUTI19. Fg deposition onto the urothelium could block E. coli-cell interaction, affecting IBC formation. Consistent with this, we found that Fg/fibrin levels directly affected E. coli CAUTI (Fig. 5f, g), decreasing colonization when Fg was soluble or absent while increasing colonization when fibrin accumulated. Soluble Fg and other host glycosylated proteins, including uromodulin78, may act as decoy receptors to prevent binding to urinary tract surfaces, resulting in the pathogen’s expulsion by urine flow. In contrast, fibrin accumulation on surfaces can provide a platform for E. coli adherence to counteract its clearance by urine flow.
Given how common urinary catheterization is and the rise of multidrug resistant pathogens, the CAUTI frequency is expected to keep increasing. This study not only has identified mechanisms by which catheter-induced inflammation predispose patients to development of CAUTIs but also identified host populations that will be at higher risk of a CAUTI-associated septicemia caused by the most prevalent CAUTI pathogens (Supplementary Fig. 9). In our comparative catheter proteomic analysis between human and mouse, we found our human catheters had less abundance of deposited proteins than in mouse catheters. This could be explained by stochastic nature of protein deposition due to urine flow or by catheter removal from the host. For example, patient catheter removal requires pulling the catheter through the urethra, which may randomly strip some proteins off. Despite this, the shared proteins are involved in the same host pathways, further confirming that our mouse CAUTI model has shown to faithfully recapitulate the pathophysiology of human CAUTI, suggesting that our findings have the potential to be translated for prevention and management of human CAUTI6,17,18,21,52. Leveraging these results to develop improved strategies for at-risk patient identification and to inform catheterization guidelines, will provide a higher patient quality of life and minimize the risk for complications.
Methods
Ethics statement
All animal care was consistent with the Guide for the Care and Use of Laboratory Animals from the National Research Council. The University of Notre Dame Institutional Animal Care and Use Committee approved all mouse infections and procedures as part of protocol #22-01-6971. For urine collections, all donors signed an informed consent form and protocol was approved by the Institutional Review Board of the University of Notre Dame under study #19-04-5273.
Mouse handling and husbandry
Mice used in this study were ~6-week-old female wild-type C57BL/6 mice purchased from Jackson Laboratory and National Institute of Cancer Research or mutant mice bred in Harper Cancer Research Institute (Notre Dame, Indiana). The University of Notre Dame Institutional Animal Care and Use Committee approved all mouse infections and procedures as part of protocol number 22-01-6971. All animal care was consistent with the Guide for the Care and Use of Laboratory Animals from the National Research Council.
Mouse infection models
Mice were catheterized by placing a silicone catheter in the bladder through the urethra (transurethral) and infected as previously described79. Briefly, mice were anesthetized with isoflurane (inhalation) and implanted transurethrally with a 6-mm-long silicone tubing which engulfed a 4mm-long PE tubing (Table 1). Mice were transurethrally infected with 50 μl of respective pathogen at an OD600 of 0.6 (∼2 × 107 CFU) or fungi at an OD600 of 5.0 (~1 × 106 CFU) in PBS, or only catheterized. In triple infection experiments, the final CFUs/50 μl were ∼2 × 107 CFU for bacteria and ~1 × 106 CFU for fungi. Immediately before sacrifice, blood was harvested via cheek poke and serum was extracted from whole blood (BD Microtainer, Table 1) for cytokine analysis as described below. To harvest catheters and organs, mice were sacrificed at their specified time-point by cervical dislocation after anesthesia inhalation; the bladder, kidneys, heart, spleen, liver, and if present, silicone catheter were aseptically harvested and bladders weighed. Except livers, all organs were homogenized (1 min shake, 5 min rest, 1 min shake; MP Biomedical 116005500) and plated for CFU enumeration as described below. A subset of bladders used for histology analysis were weighed, fixed and processed as described below. Homogenized samples of bladder, liver and blood serum were sent for cytokine analysis as described below. Catheters were subjected to 15 min sonication (Branson 2800, Table 1) for CFU enumeration or sent for proteomic analysis as described below using nonimplanted catheters as controls. The University of Notre Dame Institutional Animal Care and Use Committee approved all mouse infections and procedures as part of protocol number 22-01-6971. All animal care was consistent with the Guide for the Care and Use of Laboratory Animals from the National Research Council79.
Microbial strains and growth conditions
Microbe strains used in this paper include E. faecalis strains, uropathogenic E. coli, and fungal C. albicans details listed in Table 1. Unless otherwise noted, all E. faecalis strains were grown overnight in 10 mL of brain heart infusion (BHI) media (Hardy Diagnostics C5143) supplemented with 50 µg/ml of rifampicin and 50 µg/ml of fusidic acid at 37 °C in static conditions. C. albicans was grown in overnight in 10 mL of yeast-peptone-dextrose (YPD) media supplemented with 500 µg/ml of kanamycin at 37 °C in static conditions. E. coli UTI89 strain was grown in Luria-Bertani (LB) medium (MP Biomedicals) supplemented in 100 µg/ml of kanamycin under shaking at 37 °C for 4 h. Then, diluted in LB+kan (1:1000) then grown for 24 h in static conditions, then diluted once more in LB+kan (1:1000) and grown again for 24 h in static conditions. For differential selection and growth of the pathogens, Candida albicans was grown in YPD agar plates containing 500 µg/ml of kanamycin, a concentration that will exclude both E. faecalis and E. coli growth. C. albicans grows very well in YPD media due its acidic pH, and C. albicans growth is restricted on LB and BHI media. E. faecalis was selected on BHI plates supplemented with 50 µg/ml of rifampicin and fusidic acid; these antibiotic concentrations inhibit E. coli growth. E. faecalis growth is restrictive in YPD and LB media. Verification of the strains was further confirmed by their distinctive colony morphologies.
Human urine collection
Human urine was collected and pooled from a minimum of two healthy female donors between the ages of 20–35 years to minimize donor variability. Donor health was indicated through lack of kidney disease, diabetes mellitus, or recent antibiotic treatment. Urine was filter-sterilized with a 0.22 μm filter (VWR 29186-212) and normalized to a pH of 6.0–6.5. All participants signed an informed consent form and protocols were approved by the local Internal Review Board at the University of Notre Dame under study #19-04-5273.
Collection of human urinary catheters
Patient catheters were collected with informed consent after the clinical decision to remove for standard of care was made as described80. This study was approved by the Washington University School of Medicine (WUSM) Internal Review Board (approval #201410058) and performed in accordance with WUSM’s ethical standards and the 1964 Helsinki declaration and its later amendments. Moreover, 10 catheters that had a dwell time of 24–26 h were further processed by the S.J.H. and M.G.C. laboratories for proteomics analysis.
H&E staining of mouse bladders and imaging
Mouse bladders were fixed in 10% neutralized formalin for 18 h, embedded, sectioned, and stained as previously described21. Briefly, bladder sections were deparaffinized, rehydrated, and rinsed with water. Hematoxylin and Eosin (H&E) stain for light microscopy was done by the CORE facilities at the University of Notre Dame (ND CORE). All imaging was done using a Zeiss Axio Observer inverted light microscope. Zen Pro and ImageJ software were used to analyze the images.
Microbial enumeration
Microbe load from bladder, kidney, spleen, hearts and silicone catheters from sacrificed animals was determined via serial dilution and enumeration. Organs were homogenized and silicone catheters were cut in small pieces before sonicated for CFU enumeration. Pathogens were plated in their corresponding media conditions (see Microbial strains and growth conditions).
Cytokine analysis
Bladder, liver, and blood serum samples from mice catheterized and infected with E. faecalis OG1RF or catheterized and mock infected with PBS for 1 h, 3 h, 6 h, 9 h, 12 h, 1d, 3d, 7d, and 14d were frozen at −80 °C until time of assay. Before cytokine analysis, homogenates were thawed on ice and microcentrifuge at 11,000 × g for 10 min, and supernatants transferred to a new tube. Human urine was collected from patients catheterized for about 24 h. Both mice and human samples were probed for IL-6, IL-1α, IL-1β, and TNFα levels using a Bio-Plex Multi-Plex Assay Kit from Bio-Rad Laboratories (Table 1) following the manufacturer’s protocols.
Proteomic analysis of catheters
Donated catheters from patients catheterized for 24–26 h, or harvested catheters from mice that were catheterized and infected for 1 h, 3 h, 6 h, 1 day, 3 days, 7 days, and 14 days were boiled in SDS-sample buffer to release proteins. Protein samples were then processed by Proteomics and Mass Spectrometry Facility at the Danforth Plant Science Center (St. Louis, MO) as previously described57. Briefly, protein samples were trypsin-digested overnight at 37 °C. The extracted peptides from each sample were dried and resuspended in 100 µL 1% ACN/1% FA. 5 µL were processed with an LTQ-Orbitrap Velos Pro on a Dionex RSLCnano HPLC using a 1 h gradient. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.5.1.0). Mascot parameters were set to search the cRAP_20110301 and NCBInr databases (selected for Mus musculus or Homo sapiens depending on catheter host) with a trypsin-digestion assumption. MS/MS peptide and protein identifications were validated with Scaffold (version Scaffold_4.4.5 Proteome Software Inc., Portland, OR). Protein identifications of mouse and human catheters that were for 1 dpi (mice) and 24–26 h dwell time (humans) were compared using an E Venn network81 online software (http://www.ehbio.com/test/venn/#/) to identify common proteins in the catheterized bladder environment between mice and humans. The 76-shared proteins were then submitted to metascape.org (Supplementary Table 1) for gene ontology classification, gene ontology enrichment, protein-protein interaction identification, network analysis, and prediction of transcriptional regulatory relationships34. Generated networks were modified using Cytoscape software82.
Bacterial protease activity assay
Cultures of E. faecalis strains were centrifuged for 10 min at 3000× and washed three times with 1× PBS. Pellets were resuspended in 10 ml of filter sterilized human urine at pH 6.5. The resuspended cultures were statically incubated for 24 h at 37 °C. Following incubation, cultures were centrifuged and the supernatants were collected, filter-sterilized, and concentrated and size excluded using Macrosep Advance Centrifugal Devices (10k molecular weight cut-off; PALL MAP010C37). 40 µg/mL of purified protein (hPlasmin, hPlasminogen, hThrombin, hEndoplasmin (HSP90b1), hPrekallikrein, or hFactor XII) were added to supernatants and incubated statically for 4 h at 37 °C. To determine proteolytic activity of E. faecalis strains in mice, urine-cultured supernatants were also added to PGB mouse bladder homogenates (1:5) that were catheterized only for 24 h. The mixture was incubated for 4 h at 37 °C. Following incubation, 5× SDS sample buffer was diluted to 1× into the samples and boiled at 95 °C for 2 min before separation on 12% acrylamide gels at 100 V. Plasmin that was incubated with E. faecalis mutant strains’ supernatants was subsequently incubated with 25 µl of thrombin-generated fibrin (from 2 mg/mL of purified fibrinogen) or 25 µl of 24-h-post catheterization (hpc) Pg−/− bladder homogenates for 4 h at 37 °C to determine functional disruption of fibrinolytic activity. Fibrin and homogenates incubated with supernatant-treated plasmin was then also processed for acrylamide gel protein separation. Graphical methods provided in Supplementary Fig. 7. Normalization and western blots were done as described below.
Western blot
Western blotting was done as previously described83. Briefly, bladder homogenate samples were diluted 1:1 into 5X sample buffer then boiled at 95 °C for 2 min. Following boiling, 10 uL of sample in SDS was run on a 12% polyacrylamide gel for separation. Gels were then stained with Coomassie Blue for 60 min followed by de-staining and imaging on an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) to normalize protein concentrations. Following normalization, samples were again run on 12% acrylamide gels at 4 °C and 50 V. The samples were then transferred to polyvinylidene difluoride membrane using a semi-dry transfer (Millipore Sigma Cat# IPFL00005, Table 1). Following transfer, membranes were blocked in 5% skim milk and probed by Western immunoblot with anti-β-actin (Abcam ab8229; 1:10,000) as a loading control, and anti-plasminogen primary antibodies (Proteintech 17462-1-AP; 1:1000, respectively), anti-thrombin (ThermoFisher Scientific PA5-99213; 1:1000) or anti-Fg (Abcam ab34269; 1:1000) diluted in 3% skim milk in Tris-buffered saline-Tween (.2%). Membranes were then probed with secondary antibodies Donkey anti-goat (LI-COR Biosciences 925-68074) and Donkey anti-rabbit (LI-COR Biosciences 926-32213), diluted in dilution buffer (3% skim milk in .2% TBS-T, and .01% SDS) and visualized on an Odyssey CxL.
Measurement of Fg and IL-6 from bladder homogenates
Bladder homogenates from respective mouse bladder groups were analyzed to determine abundances of Fg and IL-6 cytokine with respective ELISA kits following manufacture-provided protocols. Briefly, bladder homogenates were centrifuged and supernatants were diluted 1:10 in ELISA diluent. Dilution standard preparation was performed following manufacturer guidelines. Standards/samples were then placed on to pre-coated ELISA plates overnight at 4 °C, removed, plates washed three times with wash buffer, incubated with detection antibodies for 1 h at room temperatures, washed three times, incubated with appropriate biotinylated-detecting enzyme for 30 min at room temperature, washed 5 times, and incubated with appropriate chromogenic substrate for 15 min at room temperature, and stopped with appropriate stop solution. Absorbances were measured at appropriate wavelengths with a plate reader (Molecular Devices Spectramax ABS plus).
Measurement of fragment E
Fragment E was probed via SDS-PAGE and western blot with an anti-Fg (1:1000) primary antibody and anti-β-actin (1:10,000) as a loading control. Fragment E probing was performed for the following experiments: i) in vitro bacterial protease activity of plasmin, previously incubated with urine-grown E. faecalis supernatant, incubated with fibrin or with Pg−/− homogenates (Fig. 4j–m), ii) homogenized bladders from both TXA-treated and vehicle controls (Fig. 6h), and iii) in vitro inhibition of plasmin fibrinolytic activity by TXA (Fig. 6i). Fragment E band was visible at ~41 kDa. Measurements of the median fluorescent intensity from each sample of both β-actin and fragment E lane were taken using image studio to determine densitometry. Measurements were graphed into Graphpad Prism 9 for statistical analysis.
Statistical analysis and reproducibility
Data derived from this study was entered into Graphpad Prism 9 to generate statistical results and graphs. Kolmogorov–Smirnov analytical tests and quantile-quantile (QQ) plot visual normality tests were performed to assess data distributions which, based on results, determined the appropriate parametric or non-parametric test to use. Normally distributed data was tested for difference between two groups with a student T-test, while an ANOVA followed by a Tukey’s post-hoc was used to determine statistical differences between more than two groups. For non-normal distributions, a Mann–Whitney U test was used to determine statistical difference between two groups, while a Kruskal–Wallis test followed by a Dunn’s test was used to determine significance between more than two groups. Pearson’s correlation statistical analysis was used to measure association between variables. Statistical tests used are indicated in figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Proteomics raw data are available in Supplementary Data 1 and 2 files. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD050199 and 10.6019/PXD050199. The data generated in this study are provided in the Source Data file. Source data are provided with this paper.
References
Haider, M. Z. & Annamaraju, P. Bladder Catheterization. In StatPearls, https://pubmed.ncbi.nlm.nih.gov/32809583/ (2022).
Armbruster, C. E., Brauer, A. L., Humby, M. S., Shao, J. & Chakraborty, S. Prospective assessment of catheter-associated bacteriuria clinical presentation, epidemiology, and colonization dynamics in nursing home residents. JCI Insight 6, e144775 (2021).
Andersen, M. J. & Flores-Mireles, A. L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 10, 23 (2020).
Nicolle, L. E. Catheter associated urinary tract infections. Antimicrobial Resist. Infect. Control 3, 23 (2014).
Manohar, J. et al. Profiles of the bacterial community in short-term indwelling urinary catheters by duration of catheterization and subsequent urinary tract infection. Am. J. Infect. Control 48, 178–183 (2020).
Flores-Mireles, A., Hreha, T. N. & Hunstad, D. A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. 25, 228–240 (2019).
Parker, D. et al. Nursing interventions to reduce the risk of catheter-associated urinary tract infection. Part 1: Catheter selection. J. Wound Ostomy Cont. Nurs. 36, 23–34 (2009).
Feneley, R. C., Hopley, I. B. & Wells, P. N. Urinary catheters: history, current status, adverse events and research agenda. J. Med. Eng. Technol. 39, 459–470 (2015).
Wagenlehner, F. M. et al. Diagnosis and management for urosepsis. Int. J. Urol. 20, 963–970 (2013).
Warren, J. W. Catheter-associated urinary tract infections. Infect. Dis. Clin. North. Am. 11, 609–622 (1997).
Trautner, B. W. & Darouiche, R. O. Catheter-associated infections: pathogenesis affects prevention. Arch. Internal Med. 164, 842–850 (2004).
CDC. Antibiotic resistance threats in the United States. U.S. Department of Health and Human Services, Center for Disease Control and Prevention https://doi.org/10.15620/cdc:82532 (2019).
Organization, W. H. Antibacterial Agents in preclinical development. (WHO, 2019).
Kattula, S., Byrnes, J. R. & Wolberg, A. S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 37, e13–e21 (2017).
Chapin, J. C. & Hajjar, K. A. Fibrinolysis and the control of blood coagulation. Blood Rev. 29, 17–24 (2015).
Flores-Mireles, A. L., Pinkner, J. S., Caparon, M. G. & Hultgren, S. J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 6, 254ra127 (2014).
Flores-Mireles, A. L. et al. Fibrinogen Release and Deposition on Urinary Catheters Placed during Urological Procedures. J. Urol. 196, 416–421 (2016).
Flores-Mireles, A. L. et al. Antibody-Based Therapy for Enterococcal Catheter-Associated Urinary Tract Infections. mBio 7, e01653–16 (2016).
Andersen, M. J. et al. Inhibiting host-protein deposition on urinary catheters reduces associated urinary tract infections. Elife 11, e75798 (2022).
La Bella, A. A. et al. The catheterized bladder environment promotes Efg1- and Als1-dependent Candida albicans infection. Sci. Adv. 9, eade7689 (2023).
Walker, J. N. et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl Acad. Sci. USA 114, E8721–E8730 (2017).
Di Venanzio, G. et al. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat. Commun. 10, 2763 (2019).
Gaston, J. R. et al. Enterococcus faecalis Polymicrobial Interactions Facilitate Biofilm Formation, Antibiotic Recalcitrance, and Persistent Colonization of the Catheterized Urinary Tract. Pathogens 9, 835 (2020).
Conway, L. J., Carter, E. J. & Larson, E. L. Risk Factors for Nosocomial Bacteremia Secondary to Urinary Catheter-Associated Bacteriuria: A Systematic Review. Urol. Nurs. 35, 191–203 (2015).
Melzer, M. & Welch, C. Outcomes in UK patients with hospital-acquired bacteraemia and the risk of catherter-associated uurinary tract infections. Postgrad. Med. J. 89, 329–334 (2013).
Melzer, M. & Welch, C. Does the presence of a urinary catheter predict severe spesis in a bacteraemic cohort? J. Hosp. Infec. 95, 376–382 (2017).
Rousseau, M. et al. Bladder catheterization increases susceptibility to infection that can be prevented by prophylactic antibiotic treatment. JCI Insight 1, e88178 (2016).
Guiton, P. S., Hannan, T. J., Ford, B., Caparon, M. G. & Hultgren, S. J. Enterococcus faecalis overcomes foreign body-mediated inflammation to establish urinary tract infections. Infect. Immun. 81, 329–339 (2013).
Xu, W. et al. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes 3, 28 (2017).
Sorensen-Zender, I. et al. Role of fibrinogen in acute ischemic kidney injury. Am. J. Physiol. Renal Physiol. 305, F777–F785 (2013).
Zandbergen, F. & Plutzky, J. PPARalpha in atherosclerosis and inflammation. Bioch. Biophys. Acta 1771, 972–982 (2007).
Yu, Y. et al. Urethral Catheter Biofilms Reveal Plasticity in Bacterial Composition and Metabolism and Withstand Host Immune Defenses in Hypoxic Environment. Front. Med. 8, 667462 (2021).
Nett, J. E. et al. Host contributions to construction of three device-associated Candida albicans biofilms. Infect. Immun. 83, 4630–4638 (2015).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Ploplis, V. A. et al. A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice. Am. J. Pathol. 157, 703–708 (2000).
Prasad, J. M. et al. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood 126, 2047–2058 (2015).
Rosen, E. D. et al. Generation of genetically-altered mice producing very low levels of coagulation factor VII. Thromb. Haemostasis 94, 493–497 (2005).
Xu, H. F., Sandoval-Cooper, M., Donahue, D. L., Ploplis, V. A. & Castellino, F. J. A FVII deficiency induces cardiac fibrosis in mice resulting in systolic and diastolic dysfunction. Blood 110, 519a–519a (2007).
Ploplis, V. A. et al. Effects of Disruption of the Plasminogen Gene on Thrombosis, Growth, and Health in Mice. Circulation 92, 2585–2593 (1995).
Carmeliet, P. et al. Physiological Consequences of Loss of Plasminogen-Activator Gene-Function in Mice. Nature 368, 419–424 (1994).
Carmeliet, P. et al. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J. Clin. Invest. 92, 2756–2760 (1993).
Iwaki, T. et al. The generation and characterization of mice expressing a plasmin-inactivating active site mutation. J. Thromb. Haemost. 8, 2341–2344 (2010).
Chauncey, J. M. & Wieters, J. S. Tranexamic Acid. In StatPearls. https://pubmed.ncbi.nlm.nih.gov/30422504/ (2023).
Levy, J. H., Koster, A., Quinones, Q. J., Milling, T. J. & Key, N. S. Antifibrinolytic Therapy and Perioperative Considerations. Anesthesiology 128, 657–670 (2018).
Franchini, M. & Mannucci, P. M. The never ending success story of tranexamic acid in acquired bleeding. Haematologica 105, 1201–1205 (2020).
Wald, H. L., Ma, A., Bratzler, D. W. & Kramer, A. M. Indwelling urinary catheter use in the postoperative period - Analysis of the National Surgical Infection Prevention Project data. Arch. Surg. Chicago 143, 551–557 (2008).
Weiner, L. M. et al. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect. Control Hospital Epidemiol. 37, 1288–1301 (2016).
Szaba, F. M. & Smiley, S. T. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 99, 1053–1059 (2002).
Smiley, S. T., King, J. A. & Hancock, W. W. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 167, 2887–2894 (2001).
Werneburg, G. T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 14, 109–133 (2022).
Letica-Kriegel, A. S. et al. Identifying the risk factors for catheter-associated urinary tract infections: a large cross-sectional study of six hospitals. BMJ Open 9, e022137 (2019).
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
Zabczyk, M., Natorska, J. & Undas, A. Fibrin Clot Properties in Atherosclerotic Vascular Disease: From Pathophysiology to Clinical Outcomes. J. Clin. Med. 10, 2999 (2021).
Jilani, T. N. & Siddiqui, A. H. Tissue Plasminogen Activator. In StatPearls, https://www.ncbi.nlm.nih.gov/books/NBK507917/ (2023).
Whyte, C. S. & Mutch, N. J. uPA-mediated plasminogen activation is enhanced by polyphosphate. Haematologica 106, 522–531 (2021).
Baker, S. K. & Strickland, S. A critical role for plasminogen in inflammation. J. Exp. Med. 217, e20191865 (2020).
Undas, A. & Ariens, R. A. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 31, e88–e99 (2011).
Franchini, M., Zaffanello, M. & Mannucci, P. M. Bleeding Disorders in Primary Fibrinolysis. Int. J. Mol. Sci. 22, 7027 (2021).
Lamers, M. M. & Haagmans, B. L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 20, 270–284 (2022).
Choi, G. et al. Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur. Respir. J. 24, 786–789 (2004).
Doherty, T. M. & Kelley, A. Bleeding Disorders. In StatPearls, https://www.ncbi.nlm.nih.gov/books/NBK541050/ (2023).
Gilliot, S. et al. Pharmacokinetics of Curative Tranexamic Acid in Parturients Undergoing Cesarean Delivery. Pharmaceutics 14, 578 (2022).
Wu, T. B. et al. Computational model of tranexamic acid on urokinase mediated fibrinolysis. PLoS One 15, e0233640 (2020).
Picetti, R., Shakur-Still, H., Medcalf, R. L., Standing, J. F. & Roberts, I. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul. Fibrinol. 30, 1–10 (2019).
Luig, M. et al. Inflammation-Induced IL-6 Functions as a Natural Brake on Macrophages and Limits GN. J. Am. Soc. Nephrol. 26, 1597–1607 (2015).
Su, H., Lei, C. T. & Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 8, 405 (2017).
Kerr, R., Stirling, D. & Ludlam, C. A. Interleukin 6 and haemostasis. Br. J. Haematol. 115, 3–12 (2001).
Zhang, C. et al. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 288, 1489–1499 (2013).
Otto, G., Braconier, J., Andreasson, A. & Svanborg, C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 179, 172–179 (1999).
Hunstad, D. A., Justice, S. S., Hung, C. S., Lauer, S. R. & Hultgren, S. J. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 73, 3999–4006 (2005).
Rodriguez, L. M., Robles, B., Marugan, J. M., Suarez, A. & Santos, F. Urinary interleukin-6 is useful in distinguishing between upper and lower urinary tract infections. Pediatr. Nephrol. 23, 429–433 (2008).
Schwartz, D. J., Conover, M. S., Hannan, T. J. & Hultgren, S. J. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog. 11, e1004599 (2015).
Yu, L. et al. Mucosal infection rewires TNFa signaling dynamics to skew susceptibility to recurrence. Elife 8, e46677 (2019).
Russell, S. K. et al. Uropathogenic Escherichia coli infection-induced epithelial trained immunity impacts urinary tract disease outcome. Nat. Microbiol. 8, 875–888 (2023).
Vu, H. M. et al. Group A Streptococcus-Induced Activation of Human Plasminogen Is Required for Keratinocyte Wound Retraction and Rapid Clot Dissolution. Front. Cardiovasc. Med. 8, 667554 (2021).
Fukuoka, K. et al. Longer Duration of Urinary Catheterization Increases Catheter-Associated Urinary Tract Infection in PICU. Pediatr. Crit. Care Med. 19, e547–e550 (2018).
Guiton, P. S. et al. Combinatorial Small-Molecule Therapy Prevents Uropathogenic Escherichia coli Catheter-Associated Urinary Tract Infections in Mice. Antimicrob. Agents Ch. 56, 4738–4745 (2012).
Kalas, V. et al. Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions. Sci. Adv. 3, e1601944 (2017).
Conover, M. S., Flores-Mireles, A. L., Hibbing, M. E., Dodson, K. & Hultgren, S. J. Establishment and Characterization of UTI and CAUTI in a Mouse Model. J. Vis. Exp. e52892, https://doi.org/10.3791/52892 (2015).
Walker, J. N. et al. High-resolution imaging reveals microbial biofilms on patient urinary catheters despite antibiotic administration. World J. Urol. 38, 2237–2245 (2020).
Chen, T., Zhang, H., Liu, Y., Liu, Y. X. & Huang, L. EVenn: Easy to create repeatable and editable Venn diagrams and Venn networks online. J. Genet. Genom. 48, 863–866 (2021).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Legesse-Miller, A., Zhang, S., Santiago-Tirado, F. H., Van Pelt, C. K. & Bretscher, A. Regulated phosphorylation of budding yeast’s essential myosin V heavy chain, Myo2p. Mol. Biol. Cell 17, 1812–1821 (2006).
Murray, B. E. et al. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bact. 175, 5216–5223 (1993).
Mulvey, M. A., Schilling, J. D. & Hultgren, S. J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 69, 4572–4579 (2001).
Odds, F. C. et al. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbio. 42, 3475–3482 (2004).
Acknowledgements
We thank the Freimann Life Science Center for mouse breeding and husbandry. Graphical illustrations were created with biorender.com. This work was supported by institutional funds from the University of Notre Dame (to A.L.F.M.), from Good Venture Foundation (Open Philanthropy) grant (to A.L.F.M., J.J.M, K.K., and M.J.A.), National Institutes of Health grants R01DK128805 (to A.L.F.M., J.J.M, K.K., and M.J.A.), R21AI163825 and U19AI157797 (to M.G.C and W.X.); R01-HL013423 (to D.D., V.A.P., and F.J.C.); R01-HL160046 and U01-HL143403 (to M.J.F); and DK051406 (to A.K., K.B., A.D, and U.D., and S.J.H). Additionally, J.J.M. has received support from the College of Science’s Paul F. Ware, M.D. Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
A.L.F.M. conceived and supervised the research. J.J.M., K.K., M.J.A., C.G., E.W., E.R.L., W.X., A.P., and A.L.F.M. conducted the experiments and sample and data analyses. A.L.F.M., A.K., K.B., A.D. conducted human sample collection. A.L.F.M., J.J.M., K.K., and M.J.A. wrote the manuscript. A.L.F.M., J.J.M., K.K., M.J.A., A.D., W.X., D.D., M.G.C., F.J.C., V.P., M.J.F., and S.J.H. reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Molina, J.J., Kohler, K.N., Gager, C. et al. Fibrinolytic-deficiencies predispose hosts to septicemia from a catheter-associated UTI. Nat Commun 15, 2704 (2024). https://doi.org/10.1038/s41467-024-46974-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46974-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.