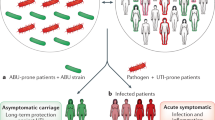
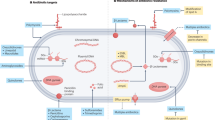
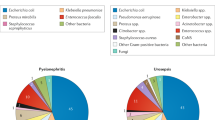
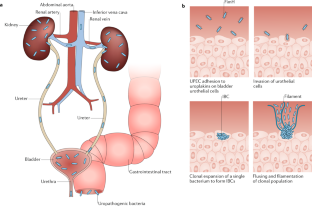
Abstract
Urinary tract infections (UTIs) are common, recurrent infections that can be mild to life-threatening. The continued emergence of antibiotic resistance, together with our increasing understanding of the detrimental effects conferred by broad-spectrum antibiotic use on the health of the beneficial microbiota of the host, has underscored the weaknesses in our current treatment paradigm for UTIs. In this Review, we discuss how recent microbiological, structural, genetic and immunological studies have expanded our understanding of host–pathogen interactions during UTI pathogenesis. These basic scientific findings have the potential to shift the strategy for UTI treatment away from broad-spectrum antibiotics targeting conserved aspects of bacterial replication towards pathogen-specific antibiotic-sparing therapeutics that target core determinants of bacterial virulence at the host–pathogen interface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Foxman, B., Barlow, R., D'Arcy, H., Gillespie, B. & Sobel, J. D. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10, 509–515 (2000).
Foxman, B. & Brown, P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. North. Am. 17, 227–241 (2003).
Scholes, D. et al. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 182, 1177–1182 (2000).
Epp, A. et al. Recurrent urinary tract infection. J. Obstet. Gynaecol. Can. 32, 1082–1090 (2010).
Foxman, B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North. Am. 28, 1–13 (2014).
Echols, R. M., Tosiello, R. L., Haverstock, D. C. & Tice, A. D. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin. Infect. Dis. 29, 113–119 (1999).
Hooton, T. M. & Gupta., K. Acute Simple Cystitis in Women (eds Calderwood, S. B. & Bloom, A.) (UpToDate, 2018).
Hooton, T. M. Clinical practice. Uncomplicated urinary tract infection. N. Engl. J. Med. 366, 1028–1037 (2012).
Katchman, E. A. et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am. J. Med. 118, 1196–1207 (2005).
Hsu, D. D. & Melzer, M. Strategy to reduce E. coli bacteraemia based on cohort data from a London teaching hospital. Postgrad. Med. J. 94, 212–215 (2018).
Seymour, C. W. et al. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 376, 2235–2244 (2017).
Hatfield, K. M. et al. Assessing variability in hospital-level mortality among U.S. Medicare beneficiaries with hospitalizations for severe sepsis and septic shock. Crit. Care Med. 46, 1753–1760 (2018).
Flores-Mireles, A. L. et al. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J. Urol. 196, 416–421 (2016). This study illustrates the molecular mechanism by which fibrinogen deposition on urinary catheters facilitates bladder colonization.
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7, 653–660 (2010).
Spees, A. M. et al. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. mBio 4, e00430-133 (2013).
Koves, B. et al. Benefits and harms of treatment of asymptomatic bacteriuria: a systematic review and meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur. Urol. 72, 865–868 (2017).
Yamamoto, S. et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J. Urol. 157, 1127–1129 (1997).
Mayer, B. T. et al. Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J. Infect. Dis. 212, 793–802 (2015).
Macklaim, J. M., Clemente, J. C., Knight, R., Gloor, G. B. & Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 26, 27799 (2015).
Hooton, T. M. et al. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 293, 949–955 (2005).
Hooton, T. M., Roberts, P. L. & Stapleton, A. E. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA 307, 583–589 (2012).
Schreiber IV, H. L. et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci. Transl Med. 9, eaaf12833 (2017).
Subashchandrabose, S. & Mobley, H. L. T. Virulence and fitness determinants of uropathogenic Escherichia coli. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.UTI-0015-2012 (2015).
Anderson, G. G. et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107 (2003).
Song, J. et al. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl Acad. Sci. USA 106, 14966–14971 (2009).
Schwartz, D. J., Chen, S. L., Hultgren, S. J. & Seed, P. C. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect. Immun. 79, 4250–4259 (2011).
Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A. & Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 (2007).
De Nisco, N. J. et al. Direct detection of tissue-resident bacteria and chronic inflammation in the bladder wall of postmenopausal women with recurrent urinary tract infection. J. Mol. Biol. 431, 4368–4379 (2019).
Robino, L. et al. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog. Dis. 68, 78–81 (2013).
Robino, L. et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin. Infect. Dis. 59, e158–e164 (2014).
Duell, B. L. et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J. Immunol. 188, 781–792 (2012).
Ingersoll, M. A., Kline, K. A., Nielsen, H. V. & Hultgren, S. J. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol. 10, 2568–2578 (2008).
Sivick, K. E., Schaller, M. A., Smith, S. N. & Mobley, H. L. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J. Immunol. 184, 2065–2075 (2010).
Schiwon, M. et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156, 456–468 (2014).
Mulvey, M. A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998).
Schaale, K. et al. Strain- and host species-specific inflammasome activation, IL-1β release, and cell death in macrophages infected with uropathogenic Escherichia coli. Mucosal Immunol. 9, 124–136 (2016).
Schlager, T. A., LeGallo, R., Innes, D., Hendley, J. O. & Peters, C. A. B cell infiltration and lymphonodular hyperplasia in bladder submucosa of patients with persistent bacteriuria and recurrent urinary tract infections. J. Urol. 186, 2359–2364 (2011).
Mysorekar, I. U. & Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA 103, 14170–14175 (2006).
Hannan, T. J. et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1, 46–57 (2014).
Hannan, T. J., Mysorekar, I. U., Hung, C. S., Isaacson-Schmid, M. L. & Hultgren, S. J. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 6, e1001042 (2010).
Ferry, S. A., Holm, S. E., Stenlund, H., Lundholm, R. & Monsen, T. J. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand. J. Infect. Dis. 36, 296–301 (2004).
Yu, L. et al. Mucosal infection rewires TNFɑ signaling dynamics to skew susceptibility to recurrence. eLife 8, e46677 (2019).
Wurpel, D. J., Beatson, S. A., Totsika, M., Petty, N. K. & Schembri, M. A. Chaperone-usher fimbriae of Escherichia coli. PLoS One 8, e52835 (2013). This study provides an excellent overview of the phenotypic relationships of CUP pili throughout all E. coli species.
Stapleton, A. E., Stroud, M. R., Hakomori, S. I. & Stamm, W. E. The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor In vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect. Immun. 66, 3856–3861 (1998).
Dodson, K. W. et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105, 733–743 (2001).
Hung, C. S. et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 44, 903–915 (2002).
Backhed, F. et al. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277, 18198–18205 (2002).
Luterbach, C. L. & Mobley, H. L. T. Cross talk between MarR-like transcription factors coordinates the regulation of motility in uropathogenic Escherichia coli. Infect. Immun. 86, e00338-18 (2018).
Wurpel, D. J. et al. F9 fimbriae of uropathogenic Escherichia coli are expressed at low temperature and recognise Galbeta1-3GlcNAc-containing glycans. PLoS One 9, e93177 (2014).
Subashchandrabose, S. et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc. Natl Acad. Sci. USA 111, 18327–18332 (2014).
Conover, M. S. et al. Inflammation-induced adhesin-receptor interaction provides a fitness advantage to uropathogenic E. coli during chronic infection. Cell Host Microbe 20, 482–492 (2016). This study elucidates the role of the F9 pilus adhesin FmlH in colonization of chronically infected bladders via interaction with galactose moieties exposed by inflammation.
Spaulding, C. N. et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 546, 528–532 (2017). This study highlights the efficacy of mannose analogues in the selective and simultaneous extirpation of UPEC from the bladder and gastrointestinal niches without a concomitant disruption of the beneficial microbiota.
Sauer, M. M. et al. Binding of the bacterial adhesin FimH to its natural, multivalent high-mannose type glycan targets. J. Am. Chem. Soc. 141, 936–944 (2018).
Kalas, V. et al. Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions. Sci. Adv. 3, e1601944 (2017).
Chen, S. L. et al. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl Acad. Sci. USA 106, 22439–22444 (2009).
Schwartz, D. J. et al. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc. Natl Acad. Sci. USA 110, 15530–15537 (2013).
Abraham, S. N. et al. Glycerol-induced unraveling of the tight helical conformation of Escherichia coli type 1 fimbriae. J. Bacteriol. 174, 5145–5148 (1992).
Aprikian, P. et al. The bacterial fimbrial tip acts as a mechanical force sensor. PLoS Biol. 9, e1000617 (2011).
Mortezaei, N. et al. Structure and function of enterotoxigenic Escherichia coli fimbriae from differing assembly pathways. Mol. Microbiol. 95, 116–126 (2015).
Spaulding, C. N. et al. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. eLife 7, e31662 (2018).
Hospenthal, M. K. et al. The cryoelectron microscopy structure of the Type 1 chaperone-usher pilus rod. Structure 25, 1829–1838.e4 (2017).
Du, M. et al. Handover mechanism of the growing pilus by the bacterial outer-membrane usher FimD. Nature 562, 444–447 (2018).
Omattage, N. S. et al. Structural basis for usher activation and intramolecular subunit transfer in P pilus biogenesis in Escherichia coli. Nat. Microbiol. 3, 1362–1368 (2018).
Pinkner, J. S. et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl Acad. Sci. USA 103, 17897–17902 (2006).
Miethke, M. & Marahiel, M. A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71, 413–451 (2007).
Lopez, C. A. & Skaar, E. P. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23, 737–748 (2018).
Weichhart, T., Haidinger, M., Horl, W. H. & Saemann, M. D. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur. J. Clin. Invest. 38, 29–38 (2008).
Reigstad, C. S., Hultgren, S. J. & Gordon, J. I. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J. Biol. Chem. 282, 21259–21267 (2007).
Patras, K. A. et al. Augmentation of urinary lactoferrin enhances host innate immune clearance of uropathogenic Escherichia coli. J. Innate Immun. 11, 481–495 (2019).
Chaturvedi, K. S., Hung, C. S., Crowley, J. R., Stapleton, A. E. & Henderson, J. P. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 8, 731–736 (2012).
Robinson, A. E., Heffernan, J. R. & Henderson, J. P. The iron hand of uropathogenic Escherichia coli: the role of transition metal control in virulence. Future Microbiol. 13, 745–756 (2018).
Henderson, J. P. et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 5, e1000305 (2009).
Johnson, J. R. et al. Contribution of yersiniabactin to the virulence of an Escherichia coli sequence type 69 (‘‘clonal group A’’) cystitis isolate in murine models of urinary tract infection and sepsis. Microb. Pathog. 120, 128–131 (2018).
Parker, K. S., Wilson, J. D., Marschall, J., Mucha, P. J. & Henderson, J. P. Network analysis reveals sex- and antibiotic resistance-associated antivirulence targets in clinical uropathogens. ACS Infect. Dis. 1, 523–532 (2015).
Koh, E. I., Robinson, A. E., Bandara, N., Rogers, B. E. & Henderson, J. P. Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat. Chem. Biol. 13, 1016–1021 (2017).
Brumbaugh, A. R. et al. Blocking yersiniabactin import attenuates extraintestinal pathogenic Escherichia coli in cystitis and pyelonephritis and represents a novel target to prevent urinary tract infection. Infect. Immun. 83, 1443–1450 (2015).
Paniagua-Contreras, G. L. et al. Comprehensive expression analysis of pathogenicity genes in uropathogenic Escherichia coli strains. Microb. Pathog. 103, 1–7 (2017).
Ohlemacher, S. I. et al. Enterobacteria secrete an inhibitor of Pseudomonas virulence during clinical bacteriuria. J. Clin. Invest. 127, 4018–4030 (2017). This study highlights the importance of metal acquisition in UPEC virulence and identifies a siderophore metabolic by-product that can inhibit iron uptake by competing bacteria.
Welch, R. A. Uropathogenic Escherichia coli-associated exotoxins. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.UTI-0011-2012 (2016).
Marrs, C. F. et al. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J. Med. Microbiol. 51, 138–142 (2002).
Hannan, T. J. et al. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol. Microbiol. 67, 116–128 (2008).
Mobley, H. L. et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58, 1281–1289 (1990).
Nagamatsu, K. et al. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl Acad. Sci. USA 112, E871–E880 (2015).
Otto, K. & Silhavy, T. J. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl Acad. Sci. USA 99, 2287–2292 (2002).
Tschauner, K., Hornschemeyer, P., Muller, V. S. & Hunke, S. Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS One 9, e107383 (2014).
Behr, S., Fried, L. & Jung, K. Identification of a novel nutrient-sensing histidine kinase/response regulator network in Escherichia coli. J. Bacteriol. 196, 2023–2029 (2014).
Steiner, B. D. et al. Evidence of cross-regulation in two closely related pyruvate-sensing systems in uropathogenic Escherichia coli. J. Membr. Biol. 251, 65–74 (2018).
Clarke, M. B. & Sperandio, V. Transcriptional autoregulation by quorum sensing Escherichia coli regulators B and C (QseBC) in enterohaemorrhagic E. coli (EHEC). Mol. Microbiol. 58, 441–455 (2005).
Breland, E. J., Zhang, E. W., Bermudez, T., Martinez, C. R. III & Hadjifrangiskou, M. The histidine residue of QseC is required for canonical signaling between QseB and PmrB in uropathogenic Escherichia coli. J. Bacteriol. https://doi.org/10.1128/JB.00060-17 (2017).
Kostakioti, M., Hadjifrangiskou, M., Pinkner, J. S. & Hultgren, S. J. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol. Microbiol. 73, 1020–1031 (2009).
Guckes, K. R. et al. Strong cross-system interactions drive the activation of the QseB response regulator in the absence of its cognate sensor. Proc. Natl Acad. Sci. USA 110, 16592–16597 (2013).
Guckes, K. R. et al. Signaling by two-component system noncognate partners promotes intrinsic tolerance to polymyxin B in uropathogenic Escherichia coli. Sci. Signal. 10, eaag1775 (2017).
Shah, C., Baral, R., Bartaula, B. & Shrestha, L. B. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 19, 204 (2019).
Eberly, A. R. et al. Biofilm formation by uropathogenic Escherichia coli is favored under oxygen conditions that mimic the bladder environment. Int. J. Mol. Sci. 18, 20777 (2017). This study reveals the mechanisms by which UPEC biofilm formation is triggered within the bladder in response to an oxygen-poor environment.
Beebout, C. J. et al. Respiratory heterogeneity shapes biofilm formation and host colonization in uropathogenic Escherichia coli. mBio 10, e02400-18 (2019).
Reichhardt, C. & Cegelski, L. Solid-state NMR for bacterial biofilms. Mol. Phys. 112, 887–894 (2013).
Chapman, M. R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002).
Van Gerven, N., Klein, R. D., Hultgren, S. J. & Remaut, H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 23, 693–706 (2015).
Biesecker, S. G., Nicastro, L. K., Wilson, R. P. & Tukel, C. The functional amyloid curli protects Escherichia coli against complement-mediated bactericidal activity. Biomolecules 8, 5 (2018).
Cegelski, L. et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 5, 913–919 (2009).
Hollenbeck, E. C. et al. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc. Natl Acad. Sci. USA 115, 10106–10111 (2018).
Klein, R. D. et al. Structure-function analysis of the curli accessory protein CsgE defines surfaces essential for coordinating amyloid fiber formation. mBio 9, e01349-18 (2018).
Evans, M. L. et al. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol. Cell 57, 445–455 (2015).
Schubeis, T. et al. Structural and functional characterization of the curli adaptor protein CsgF. FEBS Lett. 592, 1020–1029 (2018).
Sleutel, M. et al. Nucleation and growth of a bacterial functional amyloid at single-fiber resolution. Nat. Chem. Biol. 13, 902–908 (2017).
Nhu, N. T. K. et al. Discovery of new genes involved in curli production by a uropathogenic Escherichia coli strain from the highly virulent O45:K1:H7 lineage. mBio 9, e01462-18 (2018).
Smith, D. R. et al. The production of curli amyloid fibers is deeply integrated into the biology of Escherichia coli. Biomolecules 7, 75 (2017).
Majdalani, N., Heck, M., Stout, V. & Gottesman, S. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 187, 6770–6778 (2005).
Magill, S. S. et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 370, 1198–1208 (2014).
Rice, L. B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079–1081 (2008).
Santajit, S. & Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 2475067 (2016).
Weiner, L. M. et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 37, 1288–1301 (2016).
Centers for Disease Control and Prevention. 2014 HAI progress report (CDC, 2016).
Daniels, K. R., Lee, G. C. & Frei, C. R. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001–2010. Am. J. Infect. Control. 42, 17–22 (2014).
Meddings, J., Rogers, M. A., Macy, M. & Saint, S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin. Infect. Dis. 51, 550–560 (2010).
Delnay, K. M., Stonehill, W. H., Goldman, H., Jukkola, A. F. & Dmochowski, R. R. Bladder histological changes associated with chronic indwelling urinary catheter. J. Urol. 161, 1106–1108; discussion 1108–1109 (1999).
Peychl, L. & Zalud, R. Changes in the urinary bladder caused by short-term permanent catheter insertion. Cas. Lek. Cesk. 147, 325–329 (2008).
Guiton, P. S., Hannan, T. J., Ford, B., Caparon, M. G. & Hultgren, S. J. Enterococcus faecalis overcomes foreign body-mediated inflammation to establish urinary tract infections. Infect. Immun. 81, 329–339 (2013).
Flores-Mireles, A. L., Pinkner, J. S., Caparon, M. G. & Hultgren, S. J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl Med. 6, 254ra127 (2014).
Puyo, C. A. et al. Mitochondrial DNA induces Foley catheter related bladder inflammation via Toll-like receptor 9 activation. Sci. Rep. 8, 6377 (2018).
Xu, W. et al. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes 3, 28 (2017).
La Rosa, S. L., Montealegre, M. C., Singh, K. V. & Murray, B. E. Enterococcus faecalis Ebp pili are important for cell-cell aggregation and intraspecies gene transfer. Microbiology 162, 798–802 (2016).
Zhanel, G. G. et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 26, 380–388 (2005).
Al Mohajer, M., Musher, D. M., Minard, C. G. & Darouiche, R. O. Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand. J. Infect. Dis. 45, 688–695 (2013).
Gilbert, N. M. et al. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob. Adv. Health Med. 2, 59–69 (2013).
Routh, J. C., Alt, A. L., Ashley, R. A., Kramer, S. A. & Boyce, T. G. Increasing prevalence and associated risk factors for methicillin resistant staphylococcus aureus bacteriuria. J. Urol. 181, 1694–1698 (2009).
Cheng, A. G. et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23, 3393–3404 (2009).
Walker, J. N. et al. The Staphylococcus aureus ArlRS two-component system is a novel regulator of agglutination and pathogenesis. PLoS Pathog. 9, e1003819 (2013).
Walker, J. N. et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl Acad. Sci. USA 114, E8721–E8730 (2017).
McAdow, M. et al. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 7, e1002307 (2011).
Warren, J. W., Tenney, J. H., Hoopes, J. M., Muncie, H. L. & Anthony, W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146, 719–723 (1982).
Armbruster, C. E., Prenovost, K., Mobley, H. L. & Mody, L. How often do clinically diagnosed catheter-associated urinary tract infections in nursing homes meet standardized criteria? J. Am. Geriatr. Soc. 65, 395–401 (2017). In this study, the authors demonstrate the molecular basis for synergy among members of polymicrobial communities during colonization of urinary catheters in the health-care setting.
Jacobsen, S. M., Stickler, D. J., Mobley, H. L. & Shirtliff, M. E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21, 26–59 (2008).
Coker, C., Poore, C. A., Li, X. & Mobley, H. L. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2, 1497–1505 (2000).
Broomfield, R. J., Morgan, S. D., Khan, A. & Stickler, D. J. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J. Med. Microbiol. 58, 1367–1375 (2009).
Schaffer, J. N., Norsworthy, A. N., Sun, T. T. & Pearson, M. M. Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc. Natl Acad. Sci. USA 113, 4494–4499 (2016).
Armbruster, C. E. et al. The pathogenic potential of Proteus mirabilis is enhanced by other uropathogens during polymicrobial urinary tract infection. Infect. Immun. 85, e00808-16 (2017).
Shapiro, D. J., Hicks, L. A., Pavia, A. T. & Hersh, A. L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J. Antimicrob. Chemother. 69, 234–240 (2014).
Klein, T. et al. FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 53, 8627–8641 (2010).
Cusumano, C. K. et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl Med. 3, 109ra115 (2011).
Schonemann, W. et al. Improvement of aglycone pi-stacking yields nanomolar to sub-nanomolar FimH antagonists. ChemMedChem 14, 749–757 (2019).
Jarvis, C. et al. Antivirulence isoquinolone mannosides: optimization of the biaryl aglycone for FimH lectin binding affinity and efficacy in the treatment of chronic UTI. ChemMedChem 11, 367–373 (2016).
Mydock-McGrane, L. et al. Antivirulence C-mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J. Med. Chem. 59, 9390–9408 (2016).
Touaibia, M. et al. Sites for dynamic protein-carbohydrate interactions of O- and C-linked mannosides on the E. coli FimH adhesin. Molecules 22, 1101 (2017).
Kalas, V. et al. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc. Natl Acad. Sci. USA 115, E2819–E2828 (2018).
Fimbrion Therapeutics. The opportunity: mannosides as therapeutics. Fimbrion https://www.fimbrion.com/pipeline (2019).
Langermann, S. et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181, 774–778 (2000).
Savar, N. S. et al. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J. Biotechnol. 175, 31–37 (2014).
Sarkissian, C. A., Alteri, C. J. & Mobley, H. L. T. UTI patients have pre-existing antigen-specific antibody titers against UTI vaccine antigens. Vaccine 37, 4937–4946 (2019).
Mobley, H. L. & Alteri, C. J. Development of a vaccine against Escherichia coli urinary tract infections. Pathogens 5, 1 (2015).
Flores-Mireles, A. L. et al. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. mBio 7, e01653-16 (2016).
Kane, T. L., Carothers, K. E. & Lee, S. W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug. Targets 19, 111–127 (2018).
Hawkey, C. J. COX-1 and COX-2 inhibitors. Best. Pract. Res. Clin. Gastroenterol. 15, 801–820 (2001).
Bleidorn, J., Gagyor, I., Kochen, M. M., Wegscheider, K. & Hummers-Pradier, E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? - Results of a randomized controlled pilot trial. BMC Med. 8, 30 (2010).
Gagyor, I. et al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 351, h6544 (2015).
Kronenberg, A. et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ 359, j4784 (2017).
Zinkernagel, A. S., Johnson, R. S. & Nizet, V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 85, 1339–1346 (2007).
Lin, A. E. et al. Role of hypoxia inducible factor-1alpha (HIF-1alpha) in innate defense against uropathogenic Escherichia coli infection. PLoS Pathog. 11, e1004818 (2015).
Sunden, F., Hakansson, L., Ljunggren, E. & Wullt, B. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J. Urol. 184, 179–185 (2010).
Darouiche, R. O. et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 78, 341–346 (2011).
Koves, B. et al. Rare emergence of symptoms during long-term asymptomatic Escherichia coli 83972 carriage without an altered virulence factor repertoire. J. Urol. 191, 519–528 (2014).
Stork, C. et al. Characterization of asymptomatic bacteriuria Escherichia coli isolates in search of alternative strains for efficient bacterial interference against uropathogens. Front. Microbiol. 9, 214 (2018).
Hagan, E. C., Lloyd, A. L., Rasko, D. A., Faerber, G. J. & Mobley, H. L. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6, e1001187 (2010).
Chen, S. L. et al. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci. Transl Med. 5, 184ra160 (2013).
Duraj-Thatte, A. M., Praveschotinunt, P., Nash, T. R., Ward, F. R. & Joshi, N. S. Modulating bacterial and gut mucosal interactions with engineered biofilm matrix proteins. Sci. Rep. 8, 3475 (2018).
Smith, A. L. et al. Treatment and prevention of recurrent lower urinary tract infections in women: a rapid review with practice recommendations. J. Urol. 200, 1174–1191 (2018).
Dbeibo, L. et al. Evaluation of CpxRA as a therapeutic target for uropathogenic Escherichia coli infections. Infect. Immun. 86, e00798-17 (2018).
Simmering, J. E., Tang, F., Cavanaugh, J. E., Polgreen, L. A. & Polgreen, P. M. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open. Forum Infect. Dis. 4, ofw281 (2017).
Wagenlehner, F. M. et al. Diagnosis and management for urosepsis. Int. J. Urol. 20, 963–970 (2013).
O’Brien, V. P. et al. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nat. Microbiol. 2, 16196 (2016).
Hannan, T. J. et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 36, 616–648 (2012).
Sumati, A. H. & Saritha, N. K. Association of urinary tract infection in women with bacterial vaginosis. J. Glob. Infect. Dis. 1, 151–152 (2009).
Gilbert, N. M., O'Brien, V. P. & Lewis, A. L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 13, e1006238 (2017).
Stapleton, A. E. et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 52, 1212–1217 (2011).
Scholes, D. et al. Risk factors associated with acute pyelonephritis in healthy women. Ann. Intern. Med. 142, 20–27 (2005).
Bautista, C. T. et al. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil. Med. Res. 3, 4 (2016).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Touchon, M. et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5, e1000344 (2009).
Piatti, G., Mannini, A., Balistreri, M. & Schito, A. M. Virulence factors in urinary Escherichia coli strains: phylogenetic background and quinolone and fluoroquinolone resistance. J. Clin. Microbiol. 46, 480–487 (2008).
Ejrnaes, K. et al. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: phylogenetic groups, virulence factors and biofilm formation. Virulence 2, 528–537 (2011).
Wang, Y. et al. Drug resistance and virulence of uropathogenic Escherichia coli from Shanghai, China. J. Antibiot. 67, 799–805 (2014).
Nielsen, K. L. et al. Whole-genome comparison of urinary pathogenic Escherichia coli and faecal isolates of UTI patients and healthy controls. Int. J. Med. Microbiol. 307, 497–507 (2017).
Mann, R., Mediati, D. G., Duggin, I. G., Harry, E. J. & Bottomley, A. L. Metabolic adaptations of uropathogenic E. coli in the urinary tract. Front. Cell Infect. Microbiol. 7, 241 (2017).
Lavigne, J. P. et al. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: a French prospective multicentre study. J. Med. Microbiol. 65, 530–537 (2016).
Chen, Z. et al. The urinary microbiome in patients with refractory urge incontinence and recurrent urinary tract infection. Int. Urogynecol J. 29, 1775–1782 (2018).
Maddirala, A. et al. Biphenyl Gal and GalNAc FmlH Lectin antagonists of uropathogenic E. coli (UPEC): optimization through iterative rational drug design. J. Med. Chem. 62, 467–479 (2019). This study provides immunological insight into the long history of epidemiological data suggesting that a history of UTI is the greatest risk factor for the development of subsequent UTI by identifying long-term damage caused by immunomediated urothelial exfoliation.
Acknowledgements
The authors thank K. W. Dodson and T. J. Hannan for their helpful suggestions and comments on the manuscript. Work in the authors’ laboratory was supported by grants AI099099, AI095542, AI029549 and AI048689 from the US National Institution of Allergy and Infectious Diseases, grants DK051406 and DK108840 from the US National Institute of Diabetes and Digestive and Kidney Diseases and Medical Scientist Training Program Grant T32GM07200 from the US National Institute of General Medical Sciences. The authors apologize to researchers whose work was not included in this Review due to space constraints.
Author information
Authors and Affiliations
Contributions
R.D.K and S.J.H. researched data for the article, discussed the content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.J.H. has an ownership interest in Fimbrion Therapeutics, and may benefit if the company is successful in marketing mannosides. S.J.H. is also the chief scientific officer of QureTech Bio. R.D.K. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
European Association of Urology Guidelines: https://uroweb.org/wp-content/uploads/EAU-Extended-Guidelines-2015-Edn..pdf
Supplementary information
Glossary
- Urinary meatus
-
The opening of the urethra through which urine exits in males and females, sometimes referred to as the external urethral orifice.
- Uncomplicated cystitis
-
An isolated infection of the bladder and/or lower urinary tract without signs or symptoms of upper urinary tract or systemic infection in a patient without significant comorbid conditions, such as pregnancy or structural urinary tract abnormalities.
- Complicated cystitis
-
An infection of the upper urinary tract leading to upper urinary tract signs or systemic symptoms, or any urinary tract infection in pregnant women, immunocompromised patients or patients with functional urinary tract abnormalities.
- Pyelonephritis
-
An infection of the renal pelvis, calices and/or cortex.
- Fibrinogen
-
A glycoprotein released into the bladder lumen in response to inflammation and infection, and which can coat urinary catheters and serve as a nidus for bacterial binding.
- Biofilms
-
Large collections of microbial organisms embedded within a complex extracellular matrix comprising polysaccharides, proteinaceous fibres and extracellular DNA.
- Umbrella cells
-
Also known as facet cells, umbrella cells are large, polarized superficial cells that line the bladder lumen.
- C3H/HeN mice
-
An inbred mouse strain commonly used for the study of a variety of disease processes, including urinary tract infections.
- Lymphonodular hyperplasia
-
Enlargement of mucosal lymphoid nodules seen via histology.
- Bacteriuria
-
The presence of bacteria in urine not attributable to contamination. Can be symptomatic or asymptomatic.
- Nutritional immunity
-
Sequestration of nutrients by a host organism to prevent colonization by and proliferation of pathogens.
- Siderophores
-
Low molecular weight compounds secreted by the host systems to bind metal ions and transport them across cellular membranes.
Rights and permissions
About this article
Cite this article
Klein, R.D., Hultgren, S.J. Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat Rev Microbiol 18, 211–226 (2020). https://doi.org/10.1038/s41579-020-0324-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-020-0324-0
This article is cited by
-
Antibacterial properties and urease suppression ability of Lactobacillus inhibit the development of infectious urinary stones caused by Proteus mirabilis
Scientific Reports (2024)
-
Effect of DMPEI coating against biofilm formation on PVC catheter surface
World Journal of Microbiology and Biotechnology (2024)
-
Prospective Phycocompounds for Developing Therapeutics for Urinary Tract Infection
Current Microbiology (2024)
-
Predicting the primary infection source of Escherichia coli bacteremia using virulence-associated genes
European Journal of Clinical Microbiology & Infectious Diseases (2024)
-
Expert consensus on the diagnosis and treatment of end-stage liver disease complicated by infections
Hepatology International (2024)