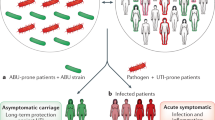
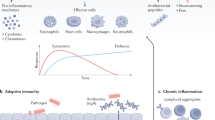
Abstract
Urinary tract infections (UTIs) are among the most common bacterial infections seen in clinical practice. The ascent of UTI-causing pathogens to the kidneys results in pyelonephritis, which can trigger kidney injury, scarring and ultimately impair kidney function. Despite sizable efforts to understand how infections develop or are cleared in the bladder, our appreciation of the mechanisms by which infections develop, progress or are eradicated in the kidney is limited. The identification of virulence factors that are produced by uropathogenic Escherichia coli to promote pyelonephritis have begun to fill this knowledge gap, as have insights into the mechanisms by which kidney tubular epithelial cells oppose uropathogenic E. coli infection to prevent or eradicate UTIs. Emerging data also illustrate how specific cellular immune responses eradicate infection whereas other immune cell populations promote kidney injury. Insights into the mechanisms by which uropathogenic E. coli circumvent host immune defences or antibiotic therapy to cause pyelonephritis is paramount to the development of new prevention and treatment strategies to mitigate pyelonephritis and its associated complications.
Key points
-
Uropathogenic Escherichia coli (UPEC) is the most common bacterial cause of pyelonephritis; virulence factors expressed by UPEC promote survival in the kidney by expediating cellular invasion and neutralizing host defences.
-
Within the collecting duct, intercalated cells are targeted by UPEC; intercalated cells respond by activating acid–base machinery, phagocytosing bacteria, producing cytokines and chemokines and releasing antimicrobial peptides.
-
An intricate network of macrophages and dendritic cells, in close proximity to collecting tubules, survey the renal interstitium and respond to UPEC by producing neutrophil and monocyte chemoattractants to combat bacteria during pyelonephritis.
-
Extracellular environmental factors and endogenous hormones influence or control important antibacterial defences in the kidney.
-
The increasing prevalence of antibiotic-resistant uropathogens highlights an urgent need for new therapeutic approaches that conserve antibiotic use and mitigate the morbidity associated with pyelonephritis; better understanding of host–pathogen interactions in the kidney may aid these efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Becknell, B., Schwaderer, A., Hains, D. S. & Spencer, J. D. Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat. Rev. Nephrol. 11, 642–655 (2015).
Butler, D. et al. Immunomodulation therapy offers new molecular strategies to treat UTI. Nat. Rev. Urol. 19, 419–437 (2022).
Mulvey, M. A., Schilling, J. D., Martinez, J. J. & Hultgren, S. J. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl Acad. Sci. USA 97, 8829–8835 (2000).
Ambite, I. et al. Molecular determinants of disease severity in urinary tract infection. Nat. Rev. Urol. 18, 468–486 (2021).
Lacerda Mariano, L. & Ingersoll, M. A. The immune response to infection in the bladder. Nat. Rev. Urol. 17, 439–458 (2020).
Morello, W., La Scola, C., Alberici, I. & Montini, G. Acute pyelonephritis in children. Pediatr. Nephrol. 31, 1253–1265 (2016).
Keren, R. et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics 136, e13–e21 (2015).
Hoberman, A. et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N. Engl. J. Med. 370, 2367–2376 (2014).
Johnson, J. R. & Russo, T. A. Acute pyelonephritis in adults. N. Engl. J. Med. 378, 48–59 (2018).
Geerlings, S. E. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int. J. Antimicrob. Agents 31, S54–S57 (2008).
Habak, P. J. & Griggs, J. R. P. Urinary Tract Infection in Pregnancy. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK537047/ (2023).
Wu, S. Y. et al. Long-term surveillance and management of urological complications in chronic spinal cord-injured patients. J. Clin. Med. https://doi.org/10.3390/jcm11247307 (2022).
Morris, B. J. & Wiswell, T. E. Circumcision and lifetime risk of urinary tract infection: a systematic review and meta-analysis. J. Urol. 189, 2118–2124 (2013).
Nordenstam, G. R., Brandberg, C. A., Oden, A. S., Svanborg Eden, C. M. & Svanborg, A. Bacteriuria and mortality in an elderly population. N. Engl. J. Med. 314, 1152–1156 (1986).
Hatfield, K. M. et al. Assessing variability in hospital-level mortality among U.S. Medicare beneficiaries with hospitalizations for severe sepsis and septic shock. Crit. Care Med. 46, 1753–1760 (2018).
Gharbi, M. et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all cause mortality: population based cohort study. Br. Med. J. 364, l525 (2019).
Wang, T. Z., Kodiyanplakkal, R. P. L. & Calfee, D. P. Antimicrobial resistance in nephrology. Nat. Rev. Nephrol. 15, 463–481 (2019).
Zowawi, H. M. et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 12, 570–584 (2015).
Desvaux, M. et al. Pathogenicity factors of genomic islands in intestinal and extraintestinal Escherichia coli. Front. Microbiol. 11, 2065 (2020).
Mobley, H. L., Donnenberg, M. S. & Hagan, E. C. Uropathogenic Escherichia coli. EcoSal https://doi.org/10.1128/ecosalplus.8.6.1.3 (2009).
Terlizzi, M. E., Gribaudo, G. & Maffei, M. E. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 8, 1566 (2017).
Wiles, T. J., Kulesus, R. R. & Mulvey, M. A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 85, 11–19 (2008).
Nielubowicz, G. R. & Mobley, H. L. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 7, 430–441 (2010). This review comprehensively outlines the UPEC virulence factors needed to establish pyelonephritis.
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Li, B. et al. Inflammation drives renal scarring in experimental pyelonephritis. Am. J. Physiol. Renal Physiol. 312, F43–F53 (2017).
Deguchi, T. et al. Electron microscopic study of acute retrograde pyelonephritis in mice. Urology 35, 423–427 (1990).
Sanford, J. P., Hunter, B. W. & Donaldson, P. Localization and fate of Escherichia coli in hematogenous pyelonephritis. J. Exp. Med. 116, 285–294 (1962).
Roy, A., Al-bataineh, M. M. & Pastor-Soler, N. M. Collecting duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 10, 305–324 (2015).
Kriz, W., Kaissling, B., Alpern, R., Caplan, M. & Moe, O. Seldin and Giebisch’s the kidney: physiology and pathophysiology. 5th edn. (eds Alpern, R. J., Moe, O.W. & Caplan M.) (Elsevier, 2013).
Saxena, V. et al. Kidney intercalated cells are phagocytic and acidify internalized uropathogenic Escherichia coli. Nat. Commun. 12, 2405 (2021). This study uses intravital microscopy and single kidney tubule perfusion to show that murine intercalated cells phagocytose UPEC to prevent pyelonephritis.
Chassin, C. et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J. Immunol. 177, 4773–4784 (2006). This landmark study identifies TLR4-dependent and -independent epithelial mechanisms that are activated in the kidney when challenged with UPEC.
Paragas, N. et al. α-Intercalated cells defend the urinary system from bacterial infection. J. Clin. Invest. 124, 2963–2976 (2014). This study shows that intercalated cell deletion increases pyelonephritis susceptibility.
McLellan, L. K. et al. A host receptor enables type 1 pilus-mediated pathogenesis of Escherichia coli pyelonephritis. PLoS Pathog. 17, e1009314 (2021).
Wu, H., Kirita, Y., Donnelly, E. L. & Humphreys, B. D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 30, 23–32 (2019).
Wu, H. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881 e868 (2018).
Korhonen, T. K., Virkola, R. & Holthofer, H. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect. Immun. 54, 328–332 (1986).
Roberts, J. A. et al. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl Acad. Sci. USA 91, 11889–11893 (1994).
Li, K., Zhou, W., Hong, Y., Sacks, S. H. & Sheerin, N. S. Synergy between type 1 fimbriae expression and C3 opsonisation increases internalisation of E. coli by human tubular epithelial cells. BMC Microbiol. 9, 64 (2009).
Springall, T. et al. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat. Med. 7, 801–806 (2001).
Chassin, C. et al. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J. Am. Soc. Nephrol. 19, 2364–2374 (2008).
Wang, C. et al. Alpha-hemolysin of uropathogenic Escherichia coli induces GM-CSF-mediated acute kidney injury. Mucosal Immunol. 13, 22–33 (2020).
Wu, J. H., Billings, B. J. & Balkovetz, D. F. Hepatocyte growth factor alters renal epithelial cell susceptibility to uropathogenic Escherichia coli. J. Am. Soc. Nephrol. 12, 2543–2553 (2001).
Trifillis, A. L. et al. Binding to and killing of human renal epithelial cells by hemolytic P-fimbriated E. coli. Kidney Int. 46, 1083–1091 (1994).
Tsuboi, N. et al. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 169, 2026–2033 (2002).
Uhlen, P. et al. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405, 694–697 (2000).
Chakrabarti, G. & McClane, B. A. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol. 7, 129–146 (2005).
Melican, K. et al. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol. 10, 1987–1998 (2008).
Kuper, C., Beck, F. X. & Neuhofer, W. Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 302, F38–F46 (2012).
Saxena, V., Arregui, S., Kamocka, M. M., Hains, D. S. & Schwaderer, A. MAP3K7 is an innate immune regulatory gene with increased expression in human and murine kidney intercalated cells following uropathogenic Escherichia coli exposure. J. Cell Biochem. 123, 1817–1826 (2022).
Hagberg, L. et al. Difference in susceptibility to Gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46, 839–844 (1984).
Patole, P. S. et al. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 68, 2582–2587 (2005).
Puthia, M. et al. IRF7 inhibition prevents destructive innate immunity — a target for nonantibiotic therapy of bacterial infections. Sci. Transl. Med. 8, 336ra359 (2016). This study shows the fine balance of the type I interferon response during pyelonephritis and the damaging effects of IRF-7 hyperactivation.
Fischer, H. et al. Pathogen specific, IRF3-dependent signaling and innate resistance to human kidney infection. PLoS Pathog. 6, e1001109 (2010).
Chowdhury, P., Sacks, S. H. & Sheerin, N. S. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin. Exp. Immunol. 145, 346–356 (2006).
Bens, M. et al. Flagellin/TLR5 signalling activates renal collecting duct cells and facilitates invasion and cellular translocation of uropathogenic Escherichia coli. Cell Microbiol. 16, 1503–1517 (2014).
Andersen-Nissen, E. et al. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 178, 4717–4720 (2007).
Hawn, T. R. et al. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS One 4, e5990 (2009).
Zhang, D. et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Tourneur, E. et al. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog. 9, e1003152 (2013).
Wang, C. et al. NOD2 is dispensable for ATG16L1 deficiency-mediated resistance to urinary tract infection. Autophagy 10, 331–338 (2014).
Saxena, V. et al. Cell specific qRT-PCR of renal epithelial cells reveals a novel innate immune signature in murine collecting duct. Am. J. Physiol. Renal Physiol. 315, F812–F823 (2017).
Saxena, V. et al. Whole transcriptome analysis of renal intercalated cells predicts lipopolysaccharide mediated inhibition of retinoid X receptor α function. Sci. Rep. 9, 545 (2019).
Zasloff, M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 18, 2810–2816 (2007).
Canas, J. J. et al. Human neutrophil peptides 1–3 protect the murine urinary tract from uropathogenic Escherichia coli challenge. Proc. Natl Acad. Sci. USA 119, e2206515119 (2022).
Chromek, M. et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12, 636–641 (2006).
Becknell, B. et al. Expression and antimicrobial function of β-defensin 1 in the lower urinary tract. PLoS One 8, e77714 (2013).
Steigedal, M. et al. Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J. Immunol. 193, 6081–6089 (2014).
Houamel, D. et al. Hepcidin as a major component of renal antibacterial defenses against uropathogenic Escherichia coli. J. Am. Soc. Nephrol. 27, 835–846 (2016).
Spencer, J. D. et al. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 83, 615–625 (2013).
Spencer, J. D. et al. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 80, 174–180 (2011).
Hains, D. S. et al. Deleted in malignant brain tumor 1 genetic variation confers urinary tract infection risk in children and mice. Clin. Transl. Med. 11, e477 (2021).
Eichler, T. et al. Ribonuclease 7 shields the kidney and bladder from invasive uropathogenic Escherichia coli infection. J. Am. Soc. Nephrol. 30, 1385–1397 (2019). This original work uses in vitro human models and a humanized transgenic mouse to show that the antimicrobial peptide RNase 7 has a role in UTI prevention.
Becknell, B. et al. Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 87, 151–161 (2015).
Jaillon, S. et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 40, 621–632 (2014).
Bender, K. et al. Expression and function of human ribonuclease 4 in the kidney and urinary tract. Am. J. Physiol. Renal Physiol. 320, F972–F983 (2021).
Bates, J. M. et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 (2004).
Pak, J., Pu, Y., Zhang, Z. T., Hasty, D. L. & Wu, X. R. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J. Biol. Chem. 276, 9924–9930 (2001).
Weiss, G. L. et al. Architecture and function of human uromodulin filaments in urinary tract infections. Science 369, 1005–1010 (2020).
Forster, C. S. et al. Urinary NGAL deficiency in recurrent urinary tract infections. Pediatr. Nephrol. 32, 1077–1080 (2017).
Eichler, T. E. et al. Insulin and the phosphatidylinositol 3-kinase signaling pathway regulate ribonuclease 7 expression in the human urinary tract. Kidney Int. 90, 568–579 (2016).
Garimella, P. S. et al. Urinary uromodulin and risk of urinary tract infections: the Cardiovascular Health Study. Am. J. Kidney Dis. 69, 744–751 (2017).
Schwaderer, A. L. et al. Polymorphisms in alpha-defensin-encoding DEFA1A3 associate with urinary tract infection risk in children with vesicoureteral reflux. J. Am. Soc. Nephrol. 27, 3175–3186 (2016).
Pierce, K. R. et al. Ribonuclease 7 polymorphism rs1263872 reduces antimicrobial activity and associates with pediatric urinary tract infections. J. Clin. Investig. https://doi.org/10.1172/JCI149807 (2021).
Murtha, M. J. et al. Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J. Clin. Invest. 128, 5634–5646 (2018). This study shows that deletion of insulin receptor in the collecting duct or intercalated cells increases UTI susceptibility by suppressing antimicrobial peptide expression.
Watts, B. A. 3rd, George, T. & Good, D. W. Lumen LPS inhibits HCO3− absorption in the medullary thick ascending limb through TLR4-PI3K-Akt-mTOR-dependent inhibition of basolateral Na+/H+ exchange. Am. J. Physiol. Renal Physiol. 305, F451–F462 (2013).
Tsuruoka, S., Purkerson, J. M. & Schwartz, G. J. Lipopolysaccharide directly inhibits bicarbonate absorption by the renal outer medullary collecting duct. Sci. Rep. 10, 20548 (2020).
Hains, D. S. et al. Carbonic anhydrase 2 deficiency leads to increased pyelonephritis susceptibility. Am. J. Physiol. Renal Physiol. 307, F869–F880 (2014).
Purkerson, J. M., Corley, J. L. & Schwartz, G. J. Metabolic acidosis exacerbates pyelonephritis in mice prone to vesicoureteral reflux. Physiol. Rep. 8, e14525 (2020).
Peng, H., Purkerson, J. M., Freeman, R. S., Schwaderer, A. L. & Schwartz, G. J. Acidosis induces antimicrobial peptide expression and resistance to uropathogenic E. coli infection in kidney collecting duct cells via HIF-1α. Am. J. Physiol. Renal Physiol. 318, F468–F474 (2020).
Peng, H., Purkerson, J. M., Schwaderer, A. L. & Schwartz, G. J. Metabolic acidosis stimulates the production of the antimicrobial peptide cathelicidin in rabbit urine. Am. J. Physiol. Renal Physiol. 313, F1061–F1067 (2017).
Ketz, J. et al. Developmental loss, but not pharmacological suppression, of renal carbonic anhydrase 2 results in pyelonephritis susceptibility. Am. J. Physiol. Renal Physiol. 318, F1441–F1453 (2020).
Hayes, B. W. & Abraham, S. N. Innate immune responses to bladder infection. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.UTI-0024-2016 (2016).
Soos, T. J. et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70, 591–596 (2006).
Weisheit, C. K., Engel, D. R. & Kurts, C. Dendritic cells and macrophages: sentinels in the kidney. Clin. J. Am. Soc. Nephrol. 10, 1841–1851 (2015). This review summarizes the classification of dendritic cells and macrophages in the kidney and their roles in pyelonephritis, acute kidney disease, chronic kidney disease and renal transplantation.
Sedin, J. et al. High resolution intravital imaging of the renal immune response to injury and infection in mice. Front. Immunol. 10, 2744 (2019).
Tittel, A. P. et al. Kidney dendritic cells induce innate immunity against bacterial pyelonephritis. J. Am. Soc. Nephrol. 22, 1435–1441 (2011).
Schiwon, M. et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156, 456–468 (2014).
Mora-Bau, G. et al. Macrophages subvert adaptive immunity to urinary tract infection. PLoS Pathog. 11, e1005044 (2015).
Berry, M. R. et al. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 170, 860–874 e819 (2017). This work demonstrates that the renal sodium stimulates NFAT5-mediated epithelial CCL2 production, which recruits mononuclear phagocytes to renal medulla and forms a medullary defence zone against uropathogens.
Ruiz-Rosado, J. D. et al. Neutrophil-macrophage imbalance drives the development of renal scarring during experimental pyelonephritis. J. Am. Soc. Nephrol. 32, 69–85 (2021). The data demonstrate that a balance between antimicrobial and inflammatory responses orchestrated by neutrophils and monocyte-derived macrophages, respectively, is required to effectively control acute pyelonephritis and prevent deteriorating kidney function.
Tsou, C. L. et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 117, 902–909 (2007).
Han, H. I., Skvarca, L. B., Espiritu, E. B., Davidson, A. J. & Hukriede, N. A. The role of macrophages during acute kidney injury: destruction and repair. Pediatr. Nephrol. 34, 561–569 (2019).
Wen, Y., Yan, H. R., Wang, B. & Liu, B. C. Macrophage heterogeneity in kidney injury and fibrosis. Front. Immunol. 12, 681748 (2021).
Haraoka, M. et al. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180, 1220–1229 (1999).
Svensson, M. et al. Acute pyelonephritis and renal scarring are caused by dysfunctional innate immunity in mCxcr2 heterozygous mice. Kidney Int. 80, 1064–1072 (2011).
Godaly, G., Proudfoot, A. E., Offord, R. E., Svanborg, C. & Agace, W. W. Role of epithelial interleukin-8 (IL-8) and neutrophil IL-8 receptor A in Escherichia coli-induced transuroepithelial neutrophil migration. Infect. Immun. 65, 3451–3456 (1997).
Javor, J. et al. Genetic variations of interleukin-8, CXCR1 and CXCR2 genes and risk of acute pyelonephritis in children. Int. J. Immunogenet. 39, 338–345 (2012).
Artifoni, L. et al. Interleukin-8 and CXCR1 receptor functional polymorphisms and susceptibility to acute pyelonephritis. J. Urol. 177, 1102–1106 (2007).
Han, S. S., Lu, Y., Chen, M., Xu, Y. Q. & Wang, Y. Association between interleukin 8-receptor gene (CXCR1 and CXCR2) polymorphisms and urinary tract infection: evidence from 4097 subjects. Nephrology 24, 464–471 (2019).
Cirl, C. et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14, 399–406 (2008). This study identifies TcpC as a UPEC-derived virulence factor that blunts TLR signalling and NF-κB activation in macrophages.
Waldhuber, A. et al. Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation. J. Clin. Investig. 126, 2425–2436 (2016). This study demonstrates that the TcpC protein blocks activation of the NLRP3 inflammasome, which serves a key role in intracellular recognition of UPEC.
Fang, J. Q. et al. TcpC inhibits Toll-like receptor signaling pathway by serving as an E3 ubiquitin ligase that promotes degradation of myeloid differentiation factor 88. PLoS Pathog. 17, e1009481 (2021). This study demonstrates that TcpC serves as an E3 ubiquitin ligase to direct the proteosomal degradation of MyD88.
Ou, Q. et al. TcpC inhibits neutrophil extracellular trap formation by enhancing ubiquitination mediated degradation of peptidylarginine deiminase 4. Nat. Commun. 12, 3481 (2021).
Bhakdi, S. et al. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J. Exp. Med. 169, 737–754 (1989).
Dhakal, B. K. & Mulvey, M. A. The UPEC pore-forming toxin α-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11, 58–69 (2012).
Verma, V. et al. α-Hemolysin of uropathogenic E. coli regulates NLRP3 inflammasome activation and mitochondrial dysfunction in THP-1 macrophages. Sci. Rep. 10, 12653 (2020).
Blomgran, R., Zheng, L. & Stendahl, O. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect. Immun. 72, 4570–4578 (2004).
Tewari, R. et al. The PapG tip adhesin of P fimbriae protects Escherichia coli from neutrophil bactericidal activity. Infect. Immun. 62, 5296–5304 (1994).
Horvath, D. J. Jr. et al. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 13, 426–437 (2011).
Justice, S. S., Hunstad, D. A., Seed, P. C. & Hultgren, S. J. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc. Natl Acad. Sci. USA 103, 19884–19889 (2006).
Stewart, B. J. et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019). This elegant study provides evidence of the spatial arrangement of immune cells in the human kidney and how it changes over developmental time and anatomical space. The results from this study suggest that antimicrobial immunity is spatially zonated but this feature is only evident postnatally.
Jobin, K. et al. A high-salt diet compromises antibacterial neutrophil responses through hormonal perturbation. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay3850 (2020). This original work demonstrates that experimental pyelonephritis is aggravated in mice on a high salt diet.
Chassin, C. et al. Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J. Exp. Med. 204, 2837–2852 (2007). This study shows that arginine vasopressin modulates antibacterial defences in the kidney.
Hale, L. J. & Coward, R. J. Insulin signalling to the kidney in health and disease. Clin. Sci. 124, 351–370 (2013).
Froy, O., Hananel, A., Chapnik, N. & Madar, Z. Differential effect of insulin treatment on decreased levels of β-defensins and Toll-like receptors in diabetic rats. Mol. Immunol. 44, 796–802 (2007).
Hiratsuka, T. et al. Structural analysis of human β-defensin-1 and its significance in urinary tract infection. Nephron 85, 34–40 (2000).
Brauner, H. et al. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin. Exp. Immunol. 177, 478–482 (2014).
Mohanty, S. et al. Diabetes downregulates the antimicrobial peptide psoriasin and increases E. coli burden in the urinary bladder. Nat. Commun. 13, 4983 (2022).
Albracht, C. D., Hreha, T. N. & Hunstad, D. A. Sex effects in pyelonephritis. Pediatr. Nephrol. 36, 507–515 (2021).
Ingersoll, M. A. Sex differences shape the response to infectious diseases. PLoS Pathog. 13, e1006688 (2017).
Zychlinsky Scharff, A. et al. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight https://doi.org/10.1172/jci.insight.122998 (2019).
Olson, P. D., Hruska, K. A. & Hunstad, D. A. Androgens enhance male urinary tract infection severity in a new model. J. Am. Soc. Nephrol. 27, 1625–1634 (2016).
Hreha, T. N. et al. Androgen-influenced polarization of activin A-producing macrophages accompanies post-pyelonephritic renal scarring. Front. Immunol. 11, 1641 (2020).
Olson, P. D. et al. Androgen exposure potentiates formation of intratubular communities and renal abscesses by Escherichia coli. Kidney Int. 94, 502–513 (2018).
Hreha, T. N. et al. TGFβ1 orchestrates renal fibrosis following Escherichia coli pyelonephritis. Physiol. Rep. 8, e14401 (2020).
Sen, A., Iyer, J., Boddu, S., Kaul, A. & Kaul, R. Estrogen receptor α differentially modulates host immunity in the bladder and kidney in response to urinary tract infection. Am. J. Clin. Exp. Urol. 7, 110–122 (2019).
Luthje, P. et al. Estrogen supports urothelial defense mechanisms. Sci. Transl. Med. 5, 190ra180 (2013).
Wang, C., Symington, J. W., Ma, E., Cao, B. & Mysorekar, I. U. Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect. Immun. 81, 733–739 (2013).
Mobley, H. L. & Alteri, C. J. Development of a vaccine against Escherichia coli urinary tract infections. Pathogens https://doi.org/10.3390/pathogens5010001 (2015).
Lorenzo-Gomez, M. F. et al. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front. Cell Infect. Microbiol. 5, 50 (2015).
Prattley, S., Geraghty, R., Moore, M. & Somani, B. K. Role of vaccines for recurrent urinary tract infections: a systematic review. Eur. Urol. Focus. 6, 593–604 (2020).
Alteri, C. J., Hagan, E. C., Sivick, K. E., Smith, S. N. & Mobley, H. L. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 5, e1000586 (2009).
Forsyth, V. S. et al. Optimization of an experimental vaccine to prevent Escherichia coli urinary tract infection. mBio https://doi.org/10.1128/mBio.00555-20 (2020).
Spurbeck, R. R. et al. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 80, 4115–4122 (2012).
Lloyd, A. L., Rasko, D. A. & Mobley, H. L. Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J. Bacteriol. 189, 3532–3546 (2007).
Langermann, S. et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181, 774–778 (2000).
Kranjcec, B., Papes, D. & Altarac, S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J. Urol. 32, 79–84 (2014).
De Nunzio, C., Bartoletti, R., Tubaro, A., Simonato, A. & Ficarra, V. Role of D-Mannose in the prevention of recurrent uncomplicated cystitis: state of the art and future perspectives. Antibiotics https://doi.org/10.3390/antibiotics10040373 (2021).
Franssen, M. et al. D-Mannose to prevent recurrent urinary tract infections (MERIT): protocol for a randomised controlled trial. BMJ Open 11, e037128 (2021).
Greene, S. E. et al. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5, e02038 (2014).
Piatek, R. et al. Pilicides inhibit the FGL chaperone/usher assisted biogenesis of the Dr fimbrial polyadhesin from uropathogenic Escherichia coli. BMC Microbiol. 13, 131 (2013).
Loubet, P. et al. Alternative therapeutic options to antibiotics for the treatment of urinary tract infections. Front. Microbiol. 11, 1509 (2020).
Sihra, N., Goodman, A., Zakri, R., Sahai, A. & Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 15, 750–776 (2018).
Pouwels, K. B., Visser, S. T., Bos, H. J. & Hak, E. Angiotensin-converting enzyme inhibitor treatment and the development of urinary tract infections: a prescription sequence symmetry analysis. Drug Saf. 36, 1079–1086 (2013).
Hall, S. A. et al. Commonly used antihypertensives and lower urinary tract symptoms: results from the Boston area community health (BACH) survey. BJU Int. 109, 1676–1684 (2012).
Blanco-Colio, L. M., Tunon, J., Martin-Ventura, J. L. & Egido, J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 63, 12–23 (2003).
Leitner, L. et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17, 90 (2017).
Ujmajuridze, A. et al. Adapted bacteriophages for treating urinary tract infections. Front. Microbiol. 9, 1832 (2018).
Zulk, J. J. et al. Phage resistance accompanies reduced fitness of uropathogenic Escherichia coli in the urinary environment. mSphere 7, e0034522 (2022).
Gu, Y. et al. Identification of novel bacteriophage vB_EcoP-EG1 with lytic activity against planktonic and biofilm forms of uropathogenic Escherichia coli. Appl. Microbiol. Biotechnol. 103, 315–326 (2019).
Pires, D. P., Melo, L., Vilas Boas, D., Sillankorva, S. & Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 39, 48–56 (2017).
Hoover, J. L., Singley, C. M., Elefante, P. & Rittenhouse, S. Efficacy of human exposures of gepotidacin (GSK2140944) against Escherichia coli in a rat pyelonephritis model. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.00086-19 (2019).
Scangarella-Oman, N. E. et al. Dose selection for phase III clinical evaluation of gepotidacin (GSK2140944) in the treatment of uncomplicated urinary tract infections. Antimicrob. Agents Chemother. 66, e0149221 (2022).
Perry, C. et al. Design of two phase III, randomized, multicenter studies comparing gepotidacin with nitrofurantoin for the treatment of uncomplicated urinary tract infection in female participants. Infect. Dis. Ther. 11, 2297–2310 (2022).
Park, J. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018).
Schwartz, L. et al. Repurposing HDAC inhibitors to enhance ribonuclease 4 and 7 expression and reduce urinary tract infection. Proc. Natl Acad. Sci. USA 120, e2213363120 (2023).
Nagamatsu, K. et al. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl Acad. Sci. USA 112, E871–E880 (2015).
Skals, M., Jorgensen, N. R., Leipziger, J. & Praetorius, H. A. Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc. Natl Acad. Sci. USA 106, 4030–4035 (2009).
Garcia, T. A., Ventura, C. L., Smith, M. A., Merrell, D. S. & O’Brien, A. D. Cytotoxic necrotizing factor 1 and hemolysin from uropathogenic Escherichia coli elicit different host responses in the murine bladder. Infect. Immun. 81, 99–109 (2013).
Mills, M., Meysick, K. C. & O’Brien, A. D. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 68, 5869–5880 (2000).
Guyer, D. M., Radulovic, S., Jones, F. E. & Mobley, H. L. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70, 4539–4546 (2002).
He, Y. et al. TcpC secreting uropathogenic E. coli promoted kidney cells to secrete MIP-2 via p38 MAPK pathway. Mol. Med. Rep. 16, 3528–3534 (2017).
Wu, X. R., Sun, T. T. & Medina, J. J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl Acad. Sci. USA. 93, 9630–9635 (1996).
Mulvey, M. A. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 4, 257–271 (2002).
Backhed, F. et al. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277, 18198–18205 (2002).
Nowicki, B., Hart, A., Coyne, K. E., Lublin, D. M. & Nowicki, S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178, 2115–2121 (1993).
Torres, A. G., Redford, P., Welch, R. A. & Payne, S. M. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69, 6179–6185 (2001).
Anderson, G. G., Goller, C. C., Justice, S., Hultgren, S. J. & Seed, P. C. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect. Immun. 78, 963–975 (2010).
Goh, K. G. K. et al. Genome-wide discovery of genes required for capsule production by uropathogenic Escherichia coli. mBio https://doi.org/10.1128/mBio.01558-17 (2017).
Corbett, D. & Roberts, I. S. The role of microbial polysaccharides in host-pathogen interaction. F1000 Biol. Rep. 1, 30 (2009).
Morrison, G., Kilanowski, F., Davidson, D. & Dorin, J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053–3060 (2002).
Valore, E. V. et al. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101, 1633–1642 (1998).
Danka, E. S. & Hunstad, D. A. Cathelicidin augments epithelial receptivity and pathogenesis in experimental Escherichia coli cystitis. J. Infect. Dis. https://doi.org/10.1093/infdis/jiu577 (2014).
Bauckman, K. A. et al. Dietary restriction of iron availability attenuates UPEC pathogenesis in a mouse model of urinary tract infection. Am. J. Physiol. Renal Physiol. 316, F814–F822 (2019).
Haversen, L. A. et al. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68, 5816–5823 (2000).
Arao, S. et al. Measurement of urinary lactoferrin as a marker of urinary tract infection. J. Clin. Microbiol. 37, 553–557 (1999).
Ghirotto, S. et al. The uromodulin gene locus shows evidence of pathogen adaptation through human evolution. J. Am. Soc. Nephrol. 27, 2983–2996 (2016).
Acknowledgements
We apologize to the authors whose important work could not be included in this article owing to space limitations. J.D.S. discloses support for publication of this work from the National Institutes of Health (NIDDK) R01 DK115737, DK114035, and DK128088 (J.D.S.). J.D.R.R. discloses support by the National Institutes of Health (NIDDK) K01 DK128379.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the manuscript and edited the final product. E.S. wrote the introduction and the section on the clinical implications of pyelonephritis; L.S. wrote the sections on antibiotic resistance and bacterial virulence factors, and created the figures; B.B. and J.D.R.R. wrote the sections on immune cells; and J.D.S. wrote the sections on epithelial responses during pyelonephritis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Michael Zasloff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schwartz, L., de Dios Ruiz-Rosado, J., Stonebrook, E. et al. Uropathogen and host responses in pyelonephritis. Nat Rev Nephrol 19, 658–671 (2023). https://doi.org/10.1038/s41581-023-00737-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00737-6
This article is cited by
-
Biomarkers for urinary tract infection: present and future perspectives
Pediatric Nephrology (2024)