Abstract
Masting, a variable and synchronized variation in reproductive effort is a prevalent strategy among perennial plants, but the factors leading to interspecific differences in masting remain unclear. Here, we investigate interannual patterns of reproductive investment in 517 species of terrestrial perennial plants, including herbs, graminoids, shrubs, and trees. We place these patterns in the context of the plants’ phylogeny, habitat, form and function. Our findings reveal that masting is widespread across the plant phylogeny. Nonetheless, reversion from masting to regular seed production is also common. While interannual variation in seed production is highest in temperate and boreal zones, our analysis controlling for environment and phylogeny indicates that masting is more frequent in species that invest in tissue longevity. Our modeling exposes masting-trait relationships that would otherwise remain hidden and provides large-scale evidence that the costs of delayed reproduction play a significant role in the evolution of variable reproduction in plants.
Similar content being viewed by others
Introduction
In perennial plants, reproduction can occur through spatially synchronized seed production, which varies substantially over time. In some years, investment in seed production is much higher than average, while in other years plants allocate few or no resources to reproduction, resulting in what is known as masting1,2. The concentration of reproduction in intermittent years appears heritable3, and helps alleviate pollen limitation and reduce seed predation but comes at the cost of skipped reproductive opportunities4,5,6,7. The varying balance of masting costs and benefits is likely responsible for the rich diversity of reproductive behaviors observed in perennials, ranging from relatively regular fruiting to rare reproduction happening at long lags1,8,9,10,11. Large-scale variation in masting benefits is better explored compared to costs1,9,11,12. For example, interannual variation in seed production is high in the temperate zone, where the benefits of starving and satiating specialist seed predators are the greatest1,13. In contrast, the costs of missed reproductive opportunities have long been only theorized to be higher in species with high population growth rates and low adult survivorship5,14, but this has remained challenging to test. Here, using trait-based approaches, we provide support for this central tenet of masting theory, showing that masting predominately occurs in species with conservative plant tissues.
Accessible trait-based approaches can serve as indicators of life history strategies, aiding in the identification of functional constraints and trade-offs15,16,17,18, and providing an avenue to investigate how varying costs of reproduction (skipped reproduction) shapes the evolution of masting. High stem tissue density (i.e. wood density) provides mechanical strength and reduces mortality, but limits growth rates, which distinguishes strategies reliant on stress persistence from rapid utilization of ephemeral opportunities17. We can thus expect stronger masting in species with high stem tissue density, as lower mortality rates due to stronger stress resistance should buffer against missed reproductive opportunities14,19,20. Similarly, productive but short-lived leaves with high nitrogen content and low leaf mass per area (LMA) are characteristic of cheap, acquisitive leaves that are efficient in resource-rich environments and associated with high population growth rates20. Such leaves should be thus associated with low interannual variation in reproduction1,21. In addition, high interannual variation should be also associated with large seeds if expensive reproduction strongly depletes resources after reproductive events5,22. Although these links are theoretically established in the literature, supporting evidence is scarce, as data on seed production accumulate slowly and require significant investment23,24.
The relationships between traits at large scales are complicated by their often-neglected direct (conditional) and indirect (marginal) relationships25,26, through the intricate connection of climate, geography, or phylogeny. In the case of masting, stem tissue density tends to be high in the tropics where interannual variation in seed production is low9,17. Therefore, a negative correlation between interannual variation in seed production and stem tissue density could be an indirect relationship resulting from latitudinal covariance in these traits. Alternatively, the relationship could be direct if the low interannual variation in seed production requires species to produce conservative stems. Indirect relationships may also arise from phylogenetic conservatism. Certain taxa may exhibit large interannual variations in seed production and high stem tissue density even if environmental conditions that select one or both traits change. Traditional summaries such as principal component analysis (PCA) summarize correlations that include all the indirect ways traits could be associated26,27. To address this issue, novel methods such as joint attribute modeling enable the decomposition of relationships into direct and indirect, driven by either climate or phylogeny26,28. These statistical tools synergize with the recent advancement of global coordination in monitoring and seed production data synthesis, allowing tests of decades-old assumptions of the field while accounting for longstanding issues with covariance between variables.
In this study, we explore the relationship between masting, phylogeny, climate, and functional diversity across 517 species of vascular plants, including herbs, graminoids, shrubs, and trees from various biomes (Fig. 1). We use MASTREE+, a database that provides information on annual variations in plant reproductive effort24. We characterize the variability of seed production in each species using two commonly used masting metrics, the coefficient of variation (CV), and the lag-1 temporal autocorrelation (AR1), which describes the tendency of high seed production years to be followed by low seed production1,29. Using joint attribute modeling, we extract conditional relationships driven by climate and phylogeny and associate large interannual variation in seed production with a need for conservative tissues. This provides large-scale evidence that the costs of delayed reproduction play a significant role in the evolution of variable reproduction.
a Location of MASTREE+ sites (red dots) included in this study (data displayed in Van der Grinten IV projection). b Climatic distribution of our sites. Each dot represents average climatic conditions (mean annual temperature, MAT, and mean annual precipitation, MAP) at the species distribution level (n = 517 species). Data on species distribution was largely derived from the Global Biodiversity Information Facility (GBIF, www.gbif.org) (see Methods). The Whittaker biome plot is included in the background for context.
Results
Masting on the spectrum of plant form
We start with results derived from the traditional principal component analysis (PCA) approach to illustrate the challenges associated with mixing conditional and marginal relationships. Principal component analysis of functional traits and masting metrics indicates that masting is largely independent of functional traits. The PCA of six functional traits and masting metrics indicated that the 517 species examined here had two primary sources of variation: an axis of leaf economics (Axis 1: leaf mass per area, leaf nitrogen, leaf area) and plant size (Axis 2: seed mass, plant height, and stem tissue density), with no contributions from masting metrics (i.e. coefficient of variation, CV, and the lag-1 of temporal auto-correlation, AR1 of seed production). Instead, masting generated a distinct axis of variation (Axis 3), with species exhibiting high CV and negative temporal autocorrelation of seed production concentrated at one end of the axis (Fig. 2 & Fig. S1). However, the correlation summary mixed conditional and marginal relationships conferred by phylogeny and climate, which each had strong effects on masting, as explained below.
a Biplot of principal components that summarized axes 1 and 2, and (b) and axes 1 and 3. The PCA included plant functional traits (stem tissue density, leaf area, leaf nitrogen, leaf mass per area LMA, plant height, and seed mass) and masting metrics (CV and AR1). Arrow length indicates the loading of each considered trait onto the axes. Points represent the position of species color-coded according to their growth form (yellow for trees, purple for shrubs, and gray for others that included graminoid and non-graminoid herbaceous and climbers). c Summary of PCA loadings, and (d) contributions to the three axes of variation. The bars at (c) and (d) are color-coded to match the colors of axes (at a, b) to which the traits loaded the most. The trait probability density function is given in Fig. S1, and CV/AR1 by growth form with PCA in Fig. S2.
Masting on the Tree of Life of plants
The coefficient of variation (CV) and the lag-1 temporal auto-correlation (AR1) exhibited phylogenetic coherence, with CV coherence being about twice as strong (CV: λ = 0.48, p < 0.0001; AR1: λ = 0.27, p < 0.0001, as shown in Fig. 3 and Fig. S3). Several groups were found to have a high concentration of species with a very high coefficient of variation in seed production (Fig. 3). These groups included Poales’ Chionochloa and Miscanthus. The Pinales order also included high-CV genera such as Abies, Juniperus, and Picea, as well as mixed ones such as Pinus. Fagales were also mixed, including high-variability genera such as Betulaceae and mixed ones such as Fagaceae, which had high-CV Fagus and diverse Quercus. Low CV was common in Magnoliales, Gentianales, and some genera of Cornales and Malvales, such as Cistaceae and Cornaceae. Highly negative temporal autocorrelation of seed production was a characteristic trait of Fagales (Fig. S3). Other groups, such as Rosales or Pinales, were mixed, while Malpighiales, Gentianales, and Magnoliales were dominated by positive autocorrelation.
Warmer colors (reds) indicate higher, while blue lower CV (the phylogenetic signal is calculated using Pagel’s λ = 0.48, p < 0.0001, n = 518 species). Distributions of the masting metrics are in Fig. S4. Orders of plants are provided at the periphery of the phylogenetic tree.
Masting across climates
Although interannual variation (CV) and lag-1 temporal auto-correlation (AR1) of seed production were not correlated (Fig. S5), they responded to the climate in opposite ways that resulted in a convergence of high CV and negative AR1 in the same climates (Fig. 4). Positive temporal autocorrelation was observed in species that grow in hot and dry environments, such as subtropical deserts or tropical seasonal forests (Fig. S6), where low CV was also common (Fig. S6). Conversely, negative AR1 and high CV were predicted in temperate and boreal forests, which are characterized by intermediate annual temperatures and precipitation (Fig. 4). We also explored models that were supplemented with climate variability (standard deviation of the monthly mean temperatures and coefficient of variation of the monthly precipitation), but the inclusion of climate variability has not improved our model’s fit (Table S2).
a Boxplots show the marginal posterior distributions of the GJAM-derived coefficients. Specifically, boxes show mean effect size as vertical lines and are bounded by 80% credible intervals (CI), with 95% CI as whiskers. Colors highlight signs of the correlation (green for positive and purple for negative), with opacity increasing from 80% to 95% of the distribution outside of zero. Gray indicates coefficients that overlap zero. b Effects of mean annual temperature (MAP, in ∘C) and mean annual precipitation (MAT, in cm) on CV and AR1. The surface shows the conditional relationship between CV/AR1 and MAT across levels of MAP. Convex hull is defined by species observations (red dots). MAT and MAP are defined for each species' distribution derived from the Global Biodiversity Information Facility (GBIF, www.gbif.org). Biplots of relationships between CV/AR1 and MAT and MAP are in Fig. S6. Climate effects on functional traits are in Fig. S7.
Masting and traits, accounted for climate and phylogeny
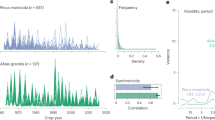
The conditional prediction from generalized joint attribute modeling (GJAM), which accounted for the effects of phylogeny and species climatic niche on masting, revealed that species with dense tissue stems and conservative leaves characterized by high mass per area tend to have higher coefficients of variation in seed production (Fig. 5). There was also a weak (non-significant in the full model) association between high CV and small seeds (Fig. 5, Table S1). These effects suggest that correlations (or the lack thereof) observed by PCA between traits and masting metrics were mainly driven by climate or shared ancestry. For instance, stem tissue density is highest in climates where masting is lowest (Fig. S7), but this negative covariance changes sign once the climate is taken into account.
Boxplots show the marginal posterior distributions of the GJAM-derived coefficients. Specifically, boxes show mean effect size as vertical lines and are bounded by 80% credible intervals (CI), with 95% CI as whiskers. Colors highlight signs of the correlation (green for positive and purple for negative), with opacity increasing from 80% to 95% of the distribution outside of zero. Gray indicates coefficients that overlap zero.
Discussion
Interannual variation in seed production across 517 species is associated with restricted climatic and phylogenetic space and conservative tissues that include higher stem tissue (wood) density and higher LMA. First, the coefficient of variation of seed production was highest in temperate and boreal climates, which supports previous studies that have shown the CV to be highest at mid-latitudes1,9. Second, masting has evolved multiple times across the Tree of Life in plants, in growth forms ranging from grasses to trees. Nonetheless, numerous branches have split into high and low-variability groups, perhaps because species quickly lose their inherited seed production variability once there is no ongoing selection for it (e.g., low seed predation or high pollination can be achieved via other routes). Third, high interannual variation in seed production is concentrated in life history strategies that invest in low mortality. High survival rates decrease the costs of missed reproductive opportunities, which is a major masting cost that can prohibit masting evolution even when there is a strong selection for it7,19. Thus, costs of delayed reproduction appear a major factor driving the evolution of masting across species.
Masting is a widespread phenomenon in the Tree of Life of plants. Although the coefficient of variation (CV) of seed production exhibited relatively strong phylogenetic coherence, branches lacking closely related species that have reverted from masting to regular seed production were rare. For instance, the Betulaceae family, comprising Betula, Carpinus, and Alnus, displayed generally high variability, with exceptions including Alnus hirsuta and Betula pendula. The closely related Chionochloa species all showed highly interannually variable seeding patterns, with related Dactylis glomerata being a low-variability exception. Perhaps the high costs of high seed production variability mean that if the need for masting (e.g., high seed predation rates or low pollination efficiency) can be circumvented through less costly alternatives, regular seeding re-evolves. In this context, oaks represent a notable example of diversity and rapid transitions between low and highly variable strategies, contrasting with Pinales, where masting was lost less frequently. A comparison of these two groups to understand why masting is almost always beneficial in Pinales, such as Picea or Abies, but can quickly cease to be so in Quercus, is a promising area for future research. Are the costs of masting systematically smaller in Pinales, or is the need for masting (e.g., low pollination efficiency) systematically greater? One interesting way forward is to examine this question in light of the high resprouting abilities of oaks but not pines30.
A high coefficient of variation in seed production does not necessarily imply a need for negative lag-1 temporal autocorrelation, indicating that the two can evolve independently9,10. High CV values without strongly negative AR1 may happen if mast years are not followed by complete failure years9,10. However, climate effects on these metrics lead to the convergence of high CV and highly negative AR1 in the same boreal and temperate habitats. Predator satiation is most effective at mid-latitudes13, which is often explained by a lower diversity of alternate food resources for seed consumers that helps control their populations1,9. Thus, the high potential effectiveness of predator satiation may lead to stronger selection for both high CV and negative AR1 in such biomes. Alternatively, species in the boreal and temperate zones may rely less on mutualistic interactions31, which tend to select against masting1,11,32. For example, wind pollination is less frequent at low latitudes33, and the absence of negative AR1 may help avoid the starvation of animal pollinators in these systems. Finally, to the extent that negative AR1 reflects resource depletion following high-seeding years34,35, convergence between high CV and negative AR1 could be driven by stronger resource constraints in certain climates21. Irrespective of the reason, in climates where high CV and negative AR1 co-occur, masting-driven pulsed resources would be expected to involve frequent famines36,37, creating an especially unstable base of food webs in these biomes.
Masting is associated with a restricted functional trait space. High interannual variation in seed production is common in species with high stem tissue density and, to a lesser extent, in species with high leaf mass per area (LMA). These species invest heavily in constructing tissues, resulting in slower returns on nutrient investment but higher survival through higher defenses against physical damage and herbivores17,38,39. Theoretical models suggest that the significant costs of missed reproductive opportunities can prevent the evolution of masting, even in the presence of significant benefits such as improved pollination and reduced seed predation7,14,19. In this context, our results support this long-standing theory, testing of which has previously been frustrated by lack of data. What is more, recent studies suggested that the other theoretical masting cost, negative density-dependent seedling survival5,40, may be lower than expected. Theory predicts that negative density-dependent seedling survival can prohibit the evolution of masting in plants that have high adult survival40. However, recent evidence implies that masting does not result in lowered seedling survival in Sorbus aucuparia12, and may even increase seeding survival in tropical communities41. Generally, negative density dependence appears fairly weak on average and highly variable among species, suggesting that its generality may be overstated42. Together with our results, these suggest that the costs of delayed reproduction may be a major mechanism driving the evolution of masting across plant life history strategies.
We also found no support for theories linking high CV with large seed5,22. We speculate that the tendency for high CV in small-seeded species, in contrast to theoretical predictions, may result from contrasting selection pressures. For example, small seeds are correlated with seed bank persistence in the soil43, which is another way to circumvent the costs of missed reproductive opportunities19. Consequently, if there are ways that small-seeded species can reduce the costs of missed reproduction, masting might evolve more readily, offsetting the expected direct effect of large seeds on masting.
In summary, our analysis supports the idea that the extent of year-to-year variation in masting is regulated by a species’ phylogeny, location (climate), and life history (plant form). The effects of climate and phylogeny on mast seeding and functional traits necessitated conditional predictions that extracted direct associations27,28. A PCA analysis that combined all the ways in which variables can be linked suggested that masting created a third, mostly independent dimension of variation in plant traits. This outcome would support a twin-filter model, according to which primary strategies, such as the fast-slow leaf economics spectrum44, determine plant persistence for climate and habitat norms, whereas traits involved in episodic events, including reproduction, affect fitness regardless of other traits45. In other words, masting would evolve whenever there is a need for it, regardless of the plant form. However, by extracting direct effects, we showed that links among traits and variation in seed production were concealed by their covariance with climate and phylogeny. That modeling reversed the analytical outcomes, showing that the costs of delayed reproduction may prevent masting in fast-growing, low-survival plant forms. The required next step is to directly link masting with life history traits (population growth rate, size at sexual maturity, mortality rates) which, with growing data availability46, may soon become feasible.
Methods
Data description
Our analysis is based on MASTREE+, a database of annual records of population-level reproductive effort of 974 from all vegetated continents24. For our analysis, we excluded time series that were on an ordinal scale and those based on pollen measurements. We analyzed two subsets of the data. One, broader, was limited to time series with at least 5 years of observations. That analysis is reported in the main text. Second, a more restrictive analysis included time series with at least 10 years of records. Results of that analysis are reported in the Supplementary Section and provide quantitatively the same outcomes.
Masting metrics
We computed the coefficient of variation (CV, standard deviation divided by mean of seed production) for each site-species combination. The CV is commonly used in masting studies to describe inter-annual variations of seed production1,29,47. We also computed lag-1 temporal auto-correlation of seed production (AR1), which characterized the tendency of high-seeding years to be followed by low-seeding years. For each species, we computed the average CV and AR1. To compute auto-regressive correlation we used the acf function in R48.
Functional traits
We extracted species-level functional traits from49, which include Leaf Mass Area (LMA, in g.m−2), stem tissue density (SSD, in mg.mm−3), plant height (ph, in m), leaf nitrogen (ln, in mg.g−1), seed size (sm, in mg), and leaf area (la, in mm−2), and plant growth form (Fig. S8). We obtained plant growth form, which includes trees, shrubs, and other categories, with graminoid and non-graminoid herbaceous, and climbers (see distribution in Fig. S2).
Full trait information obtained from49 was available for 210 species from MASTREE+ database. To increase species coverage, we performed a trait-imputation procedure. We used machine learning that accounted for species phylogeny50,51. We filled only species that had information for at least three functional traits (out of six used in the analysis)18,50. First, we log10 transformed known functional traits and incorporated phylogenetic information for each species52. The phylogenetic information was summarized by eigenvectors extracted from a principal coordinate analysis (PCoA), which represented the variation in the phylogenetic distances among species. We used the first ten axes of PCoA for the imputation process50,52. The phylogeny was obtained using the R package V.Phylomaker253,54, with the GBOTB.extented.TPL tree as a backbone55,56, and scenario S3 to generate the phylogeny54,57. Imputation of missing trait information with machine learning has been done through the R package missForest58. That imputation allowed us to increase the sample size (i.e. species for which we had full traits and seed production data) to 517 species. The GJAM model without trait data imputation generated qualitatively similar results for CV (Table S1). In the case of AR1, lack of trait imputation resulted in a positive association between leaf N and AR1, and a negative between height and AR1 being significant. That hints that acquisitive leaves may buffer against strong post-mast seeding failure21, although it is unclear why smaller plants have more negative AR1. For consistency, we discuss only the results with the data imputation in the main text.
Abiotic variables
We determined the species’ climatic niche by using species occurrences extracted from Global Biodiversity Information Facility (GBIF, www.gbif.org) through the rgbif package59 (data request: https://doi.org/10.15468/dl.jxyrhk)60. We removed species occurrences from GBIF that are incorrectly or vaguely reported and outliers by using the R package CoordinateCleaner61 to keep precise species locations (mean number of occurrences for our species = 7609, CI975 = [1; 105,093]). Next, for each occurrence, we extracted a mean annual temperature (MAT, in °C) and mean annual cumulative precipitation (MAP, in cm) by using CHELSA data62, and averaged those values from all occurrences per species to one value per species range (MAT and MAP). For each species, we used average species climatic conditions from MASTREE+ if the number of sample sites from MASTREE+ was higher than the number of species occurrences from GBIF (n = 55 species). We used GBIF-based climate to accommodate functional traits and masting metrics at species-wide averages. Nonetheless, MAT and MAP obtained through MASTREE+ sites and GBIF present strong correlations (Fig. S9), and using both provides qualitatively the same results.
Analysis
Phylogenetic analysis
We estimated the phylogenetic signal of the coefficient of variation (CV) and temporal auto-correlation (AR1) of seed production with Pagel’s λ63. Pagel’s λ is based on the Brownian Motion evolutionary model and ranges from 0, when there is no phylogenetic signal, to 1 where the phylogenetic signal is estimated to be very strong. The Pagel’s λ was estimated by using the phyolosig function from phytools R package64 and visualized with ggtree65. We used a plant phylogenetic tree provided by55.
Multivariate analysis
We used the principal component analysis (PCA) to describe the multivariate trait spectrum, which included the six functional traits and two masting metrics (CV and AR1). We kept functional traits log10 transformed. We standardized and centered variables. We used ade466 R package. Moreover, we estimated the occurrence probability of trait combination in two-dimensional space (determined by the PCA axis 1 and 2, or by axis 2 and 3) with their bivariate trait combinations. We used the two-dimensional kernel density estimation and determined the highest probability trait occurrence18,51.
Joint model analysis
We jointly modeled functional traits and masting metrics using the generalized joint attribute modeling (GJAM,28). Average climatic conditions per species range (occurrences obtained via GBIF, see above) were included as predictors, i.e. mean annual temperature (MAT) and mean annual precipitation (MAP). We tested a set of models with different combinations of the interaction between MAP and MAT, and their quadratic terms. Model selection was based on the Deviance information criterion (DIC). The GJAM allowed us to accommodate the dependence between traits and phylogeny as random groups. To this end, we followed past studies that used a similar approach27,67, and grouped species according to genus or family (when the genus had <10 species). We used the ’multiple’ category for families with <5 species.
We accommodated the mutual dependence structure of traits and isolated their effect on masting metrics through conditional prediction27,68. Conditional prediction offers an estimation of the relationships between traits and masting metrics while accounting for the effects that come through climate and phylogeny. These conditional parameters are obtained via gjam R package28, by specifying traits being conditioned (here, functional traits) on the variable of interest (here, CV and AR1 of seed production). In doing this, we first estimate how responses (functional traits and masting metrics) correlate with climate. Next, the relationships among responses are estimated, after accounting for the predictors (climate and phylogeny). The gjam is an open-access R package gjam available on CRAN.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used in this study have been deposited in the Open Science Framework (OSF) (https://osf.io/57w2q/). The full MASTREE+ dataset is available in24. Traits have been downloaded from49. Climate data have been extracted from CHELSA at https://chelsa-climate.org/.
Code availability
R statistical software v4.3.0 was used in this work48. All analyses used published R packages.
References
Kelly, D. & Sork, V. L. Mast seeding in perennial plants: why, how, where? Annu. Rev. Ecol. Syst. 33, 427–447 (2002).
Pesendorfer, M. B. et al. The ecology and evolution of synchronized reproduction in long-lived plants. Philos. Trans. Royal Soc. B Biol. Sci. 376, 1–8 (2021).
Caignard, T., Delzon, S., Bodénès, C., Dencausse, B. & Kremer, A. Heritability and genetic architecture of reproduction-related traits in a temperate oak species. Tree Genet. Genom. 15, 1 (2019).
Hett, J. M. A dynamic analysis of age in sugar maple seedlings. Ecology 52, 1071–1074 (1971).
Kelly, D. The evolutionary ecology of mast seeding. Trends Ecol. Evol. 9, 465–470 (1994).
Norton, D. A. & Kelly, D. Mast seeding over 33 years by dacrydium cupressinum lamb. (rimu) (podocarpaceae) in new zealand: The importance of economies of scale. Funct. Ecol. 2, 399–408 (1988).
Tachiki, Y. & Iwasa, Y. Both seedling banks and specialist seed predators promote the evolution of synchronized and intermittent reproduction (masting) in trees. J. Ecol. 98, 1398–1408 (2010).
Koenig, W. D. et al. Is the relationship between mast-seeding and weather in oaks related to their life-history or phylogeny? Ecology 97, 2603–2615 (2016).
Pearse, I. S., LaMontagne, J. M., Lordon, M., Hipp, A. L. & Koenig, W. D. Biogeography and phylogeny of masting: do global patterns fit functional hypotheses? New Phytol. 227, 1557–1567 (2020).
Dale, E. E., Foest, J. J., Hacket-Pain, A., Bogdziewicz, M. & Tanentzap, A. J. Macroevolutionary consequences of mast seeding. Philos. Transac. Royal Soc. B Biol. Sci. 376, 20200372 (2021).
Qiu, T. et al. Masting is uncommon in trees that depend on mutualist dispersers in the context of global climate and fertility gradients. Nat. Plants 9, 1044–1056 (2023).
Seget, B. et al. Costs and benefits of masting: economies of scale are not reduced by negative density-dependence in seedling survival in sorbus aucuparia. New Phytol. 233, 1931–1938 (2022).
Zwolak, R., Celebias, P. & Bogdziewicz, M. Global patterns in the predator satiation effect of masting: a meta-analysis. Proc. Natl Acad. Sci. USA 119, e2105655119 (2022).
Waller, D. M. Models of mast fruiting in trees. J. Theoret. Biol. 80, 223–232 (1979).
Violle, C. et al. Let the concept of trait be functional! Oikos 116, 882–892 (2007).
Muller-Landau, H. C. The tolerance-fecundity trade-off and the maintenance of diversity in seed size. Proc. Natl Acad. Sci. USA 107, 4242–4247 (2010).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Díaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016).
Rees, M., Kelly, D. & Bjørnstad, O. N. Snow tussocks, chaos, and the evolution of mast seeding. Am. Nat. 160, 44–59 (2002).
Adler, P. B. et al. Functional traits explain variation in plant lifehistory strategies. Proc. Natl Acad. Sci. USA 111, 740–745 (2014).
Fernández-Martínez, M. et al. Nutrient scarcity as a selective pressure for mast seeding. Nat. Plants 5, 1222–1228 (2019).
Sork, V. L., Bramble, J. & Sexton, O. Ecology of mast-fruiting in three species of North American deciduous oaks. Ecology 74, 528–541 (1993).
Clark, J. S. et al. Continent-wide tree fecundity driven by indirect climate effects. Nat. Commun. 12, 1242 (2021).
Hacket-Pain, A. et al. Mastree+: Time-series of plant reproductive effort from six continents. Glob. Change Biol. 28, 3066–3082 (2022).
Agrawal, A. A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 101, e02924 (2020).
Seyednasrollah, B. & Clark, J. S. Where resource-acquisitive species are located: the role of habitat heterogeneity. Geophys. Res. Lett. 47, 1–12 (2020).
Bogdziewicz, M. et al. Linking seed size and number to trait syndromes in trees. Glob. Ecol. Biogeogr. 32, 683–694 (2023).
Clark, J. S., Nemergut, D., Seyednasrollah, B., Turner, P. J. & Zhang, S. Generalized joint attribute modeling for biodiversity analysis: median-zero, multivariate, multifarious data. Ecol. Monograp. 87, 34–56 (2017).
Koenig, W. D. et al. Dissecting components of population-level variation in seed production and the evolution of masting behavior. Oikos 102, 581–591 (2003).
Vacchiano, G. et al. Natural disturbances and masting: from mechanisms to fitness consequences. Philos. Trans. Royal Soc. B Biol. Sci. 376, 20200384 (2021).
Schemske, D. W., Mittelbach, G. G., Cornell, H. V., Sobel, J. M. & Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (2009).
Herrera, C. M., Jordano, P., Guitian, J. & Traveset, A. Annual variability in seed production by woody plants and the masting concept: Reassessment of principles and relationship to pollination and seed dispersal. Am. Nat. 152, 576–594 (1998).
Regal, P. J. Pollination by wind and animals: ecology of geographic patterns. Annu. Rev. Ecol. Syst. 13, 497–524 (1982).
Sala, A., Hopping, K., McIntire, E. J., Delzon, S. & Crone, E. E. Masting in whitebark pine (pinus albicaulis) depletes stored nutrients. New Phytol. 196, 189–199 (2012).
Crone, E. E. & Rapp, J. M. Resource depletion, pollen coupling, and the ecology of mast seeding. Ann. New York Acad. Sci. 1322, 21–34 (2014).
Bogdziewicz, M., Zwolak, R. & Crone, E. E. How do vertebrates respond to mast seeding? Oikos 125, 300–307 (2016).
Clark, J. S., Nuñez, C. L. & Tomasek, B. Foodwebs based on unreliable foundations: spatiotemporal masting merged with consumer movement, storage, and diet. Ecol. Monogr. 89, 1–24 (2019).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
King, D. A., Wright, S. J. & Connell, J. H. The contribution of interspecific variation in maximum tree height to tropical and temperate diversity. J. Trop. Ecol. 22, 11–24 (2006).
Visser, M. D. et al. Strict mast fruiting for a tropical dipterocarp tree: a demographic cost-benefit analysis of delayed reproduction and seed predation. J. Ecol. 99, 1033–1044 (2011).
Martini, F., Chang-Yang, C. H. & Sun, I. F. Variation in biotic interactions mediates the effects of masting and rainfall fluctuations on seedling demography in a subtropical rainforest. J. Ecol. 110, 762–771 (2022).
Song, X., Lim, J. Y., Yang, J. & Luskin, M. S. When do janzen-connell effects matter? a phylogenetic meta analysis of conspecific negative distance and density dependence experiments. Ecol. Lett. 24, 608–620 (2021).
Gioria, M., Pyšek, P., Baskin, C. C. & Carta, A. Phylogenetic relatedness mediates persistence and density of soil seed banks. J. Ecol. 108, 2121–2131 (2020).
Reich, P. B. The world-wide ’fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 102, 275–301 (2014).
Pierce, S., Bottinelli, A., Bassani, I., Ceriani, R. M. & Cerabolini, B. E. How well do seed production traits correlate with leaf traits, whole-plant traits and plant ecological strategies? Plant Ecol. 215, 1351–1359 (2014).
Salguero-Gómez, R. et al. The compadre plant matrix database: an open online repository for plant demography. J. Ecol. 103, 202–218 (2015).
Pearse, I. S., Wion, A. P., Gonzalez, A. D. & Pesendorfer, M. B. Understanding mast seeding for conservation and land management. Philos. Trans. Royal Soc. B Biol. Sci. 376, 20200383 (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing Austria, 2023).
Díaz, S. et al. The global spectrum of plant form and function: enhanced species-level trait dataset. Sci. Data 9, 1–18 (2022).
Carmona, C. P. et al. Fine-root traits in the global spectrum of plant form and function. Nature 597, 683–687 (2021).
Carmona, C. P., Pavanetto, N. & Puglielli, G. funspace : an R package to build, analyze and plot functional trait spaces. Preprint 1–26 (2023).
Penone, C. et al. Imputation of missing data in life-history trait datasets: Which approach performs the best? Methods Ecol. Evol. 5, 961–970 (2014).
Jin, Y. & Qian, H. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42, 1353–1359 (2019).
Jin, Y. & Qian, H. V.PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Div. 44, 335–339 (2022).
Zanne, A. E. et al. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014).
Smith, S. A. & Brown, J. W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Botany 105, 302–314 (2018).
Qian, H. & Jin, Y. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol. 9, 233–239 (2016).
Stekhoven, D. J. & Bühlmann, P. Missforest-Non-parametric missing value imputation for mixed-type data. Bioinformatics 28, 112–118 (2012).
Chamberlain, S. & Boettiger, C. R python, and ruby clients for gbif species occurrence data. Peer J. https://doi.org/10.7287/peerj.preprints.3304v1 (2017).
Global biodiversity information facility. Occurrence data Data Retrieved from GBIF Request https://www.gbif.org/occurrence/download/0021121-230224095556074 (2023).
Zizka, A. et al. Coordinatecleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 10, 744–751 (2019).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 1–20 (2017).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401, 877–884 (1999).
Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Yu, G., Smith, D. K., Zhu, H., Guan, Y. & Lam, T. T. Y. Ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 8, 28–36 (2017).
Dray, S. & Dufour, A.-B. The ade4 package: Implementing the duality diagram for ecologists. J. Statist. Softw. 22, 1–20 (2007).
Qiu, T. et al. Limits to reproduction and seed size-number trade-offs that shape forest dominance and future recovery. Nat. Commun. 13, 2381 (2022).
Qiu, T., Sharma, S., Woodall, C. W. & Clark, J. S. Niche shifts from trees to fecundity to recruitment that determine species response to climate change. Front. Ecol. Evol. 9, 1–12 (2021).
Acknowledgements
We thank Jessie Foest for her help with extracting data from the MASTREE+ database and Kevin Sartori for early discussions and suggestions. This study was conceived during a workshop funded by the UK Natural Environment Research Council grant no. NE/S007857/1, and uses a dataset created as part of that project. VJ was supported by project No. 2021/43/P/NZ8/01209 co-funded by the Polish National Science Centre and the EU H2020 research and innovation program under the MSCA GA No. 945339. MB was supported by the European Union (ERC, ForestFuture, 101039066). Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
V.J. and M.B designed the study. V.J. led the analysis with inputs from M.B. and A. H-P. M.B. and V.J. co-wrote the first draft of the paper. Revisions were done by all Authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Communications thanks Benedicte Bachelot and Giorgio Vacchiano for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Journé, V., Hacket-Pain, A. & Bogdziewicz, M. Evolution of masting in plants is linked to investment in low tissue mortality. Nat Commun 14, 7998 (2023). https://doi.org/10.1038/s41467-023-43616-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43616-1
This article is cited by
-
Reproductive patterns in Araucaria araucana forests in the Andean range, Chile
Ecological Processes (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.