Abstract
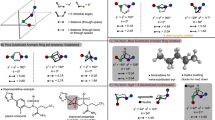
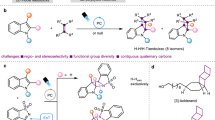
Small-ring cage hydrocarbons are popular bioisosteres (molecular replacements) for commonly found para-substituted benzene rings in drug design1. The utility of these cage structures derives from their superior pharmacokinetic properties compared with their parent aromatics, including improved solubility and reduced susceptibility to metabolism2,3. A prime example is the bicyclo[1.1.1]pentane motif, which is mainly synthesized by ring-opening of the interbridgehead bond of the strained hydrocarbon [1.1.1]propellane with radicals or anions4. By contrast, scaffolds mimicking meta-substituted arenes are lacking because of the challenge of synthesizing saturated isosteres that accurately reproduce substituent vectors5. Here we show that bicyclo[3.1.1]heptanes (BCHeps), which are hydrocarbons for which the bridgehead substituents map precisely onto the geometry of meta-substituted benzenes, can be conveniently accessed from [3.1.1]propellane. We found that [3.1.1]propellane can be synthesized on a multigram scale, and readily undergoes a range of radical-based transformations to generate medicinally relevant carbon- and heteroatom-substituted BCHeps, including pharmaceutical analogues. Comparison of the absorption, distribution, metabolism and excretion (ADME) properties of these analogues reveals enhanced metabolic stability relative to their parent arene-containing drugs, validating the potential of this meta-arene analogue as an sp3-rich motif in drug design. Collectively, our results show that BCHeps can be prepared on useful scales using a variety of methods, offering a new surrogate for meta-substituted benzene rings for implementation in drug discovery programmes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Auberson, Y. P. et al. Improving nonspecific binding and solubility: bicycloalkyl groups and cubanes as para-phenyl bioisosteres. Chem. Med. Chem. 12, 590–598 (2017).
Ma, X. & Pham, Nhat L. Selected topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 9, 8–22 (2020).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Meanwell, N. A. Improving drug design: an update on recent applications of efficiency metrics, strategies for replacing problematic elements, and compounds in nontraditional drug space. Chem. Res. Toxicol. 29, 564–616 (2016).
Chalmers, B. A. et al. Validating Eaton’s hypothesis: cubane as a benzene bioisostere. Angew. Chem. Int. Edn 55, 3580–3585 (2016).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Pu, Q. et al. Discovery of potent and orally available bicyclo[1.1.1]pentane-derived indoleamine-2,3-dioxygenase 1 (IDO1) inhibitors. ACS Med. Chem. Lett. 11, 1548–1554 (2020).
Measom, N. D. et al. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett. 8, 43–48 (2017).
Nilova, A., Campeau, L.-C., Sherer, E. C. & Stuart, D. R. Analysis of benzenoid substitution patterns in small molecule active pharmaceutical ingredients. J. Med. Chem. 63, 13389–13396 (2020).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Edn 59, 20515–20521 (2020).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Edn 59, 7161–7167 (2020).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Preprint at https://doi.org/10.26434/chemrxiv-22022-v26493kv (2022).
Guo, R. et al. Strain-release [2π + 2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes Initiated by energy transfer. J. Am. Chem. Soc. 144, 7988–7994 (2022).
Dhake, K. et al. Beyond bioisosteres: divergent synthesis of azabicyclohexanes and cyclobutenyl amines from bicyclobutanes. Angew. Chem. Int. Edn 61, e202204719 (2022).
Zhao, J. X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: long-sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl Acad. Sci. USA 118, e2108881118 (2021).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Anderson, J. M., Measom, N. D., Murphy, J. A. & Poole, D. L. Bridge functionalisation of bicyclo[1.1.1]pentane derivatives. Angew. Chem. Int. Edn 60, 24754–24769 (2021).
Harmata, A. S., Spiller, T. E., Sowden, M. J. & Stephenson, C. R. J. Photochemical formal (4 + 2)-cycloaddition of imine-substituted bicyclo[1.1.1]pentanes and alkenes. J. Am. Chem. Soc. 143, 21223–21228 (2021).
Della, E. W. & Elsey, G. M. Bridgehead carbocations: solvolysis of a series of 5-substituted bicycle [3.1.1]heptyl bromides. Nucleophilic solvent assistance to ionization of 1-bromobicyclo[3.1.1]heptane. Aust. J. Chem. 48, 967–985 (1995).
Dilmac, A. M., Spuling, E., de Meijere, A. & Brase, S. Propellanes-from a chemical curiosity to “explosive” materials and natural products. Angew. Chem. Int. Edn 56, 5684–5718 (2017).
Wiberg, K. B. Small ring propellanes. Chem. Rev. 89, 975–983 (1989).
Gassman, P. G. & Proehl, G. S. [3.1.1]Propellane. J. Am. Chem. Soc. 102, 6862–6863 (1980).
Fuchs, J. S. G. Synthese von [n.l.l]propellanen (n = 2, 3, 4). Chem. Ber. 125, 2517–2522 (1992).
Kulinkovich, O. G., Kozyrkov, Y. Y., Bekish, A. V., Matiushenkov, E. A. & Lysenko, I. L. A convenient way for the conversion of carboxylic esters into 2-substituted allyl halides. Synthesis 2005, 1713–1717 (2005).
Sterling, A. J., Dürr, A. B., Smith, R. C., Anderson, E. A. & Duarte, F. Rationalizing the diverse reactivity of [1.1.1]propellane through σ–π-delocalization. Chem. Sci. 11, 4895–4903 (2020).
Sterling, A., Smith, R., Anderson, E. & Duarte, F. Beyond strain release: delocalisation-enabled organic reactivity. Preprint at https://doi.org/10.26434/chemrxiv-22021-n26430xm26439-v26432 (2022).
Caputo, D. F. J. et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 9, 5295–5300 (2018).
Nugent, J. et al. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 9, 9568–9574 (2019).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Zarate, C. et al. Development of scalable routes to 1-bicyclo[1.1.1]pentylpyrazoles. Org. Proc. Res. Dev. 25, 642–647 (2021).
Pickford, H. D. et al. Twofold radical-based synthesis of N,C-difunctionalized bicyclo[1.1.1]pentanes. J. Am. Chem. Soc. 143, 9729–9736 (2021).
Wu, Z., Xu, Y., Wu, X. & Zhu, C. Synthesis of selenoether and thioether functionalized bicyclo[1.1.1]pentanes. Tetrahedron 76, 131692 (2020).
Bär, R. M., Kirschner, S., Nieger, M. & Bräse, S. Alkyl and aryl thiol addition to [1.1.1]propellane: scope and limitations of a fast conjugation reaction. Chem. Eur. J. 24, 1373–1382 (2018).
Bär, R. M., Heinrich, G., Nieger, M., Fuhr, O. & Bräse, S. Insertion of [1.1.1]propellane into aromatic disulfides. Beilstein J. Org. Chem. 15, 1172–1180 (2019).
Tokunaga, K. et al. Bicyclobutane carboxylic amide as a cysteine-directed strained electrophile for selective targeting of proteins. J. Am. Chem. Soc. 142, 18522–18531 (2020).
Nugent, J. et al. Synthesis of all-carbon disubstituted bicyclo[1.1.1]pentanes by iron-catalyzed Kumada cross-coupling. Angew. Chem. Int. Edn 59, 11866–11870 (2020).
Acknowledgements
N.F. thanks Studienstiftung des Deutschen Volkes e.V. for a scholarship. J.N. thanks the Marie Skłodowska-Curie actions for an Individual Fellowship (GA No 786683). E.A.A. and A.J.S. thank the EPSRC for support (grant nos EP/S013172/1 and EP/T517811/1). B.R.S., A.J.S. and H.D.P. thank the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine for studentships (EP/ L015838/1). T.Z.-T., T.G. and P.E.B. thank Alzheimer’s Research UK for support (grant no. ARUK-2021DDI-OX). P.R. thanks the Deutsche Akademie für Naturforscher Leopoldina for a fellowship.
Author information
Authors and Affiliations
Contributions
E.A.A., N.F., J.N. and A.J.S. conceived the project. Experimental work was carried out by N.F., J.N., B.R.S., P.R., T.Z.-T. and T.G. H.D.P. and A.L.T. collected the crystallographic data. N.F. and A.J.S. carried out the computational analysis. The project was supervised by E.A.A., F.D., P.E.B. and C.J.S. E.A.A., N.F., J.N. and F.D. wrote the initial manuscript which was reviewed and edited by E.A.A., N.F., J.N., R.C.S., P.E.B. and F.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Tian Qin, Antonia Stepan and the other, anonymous, reviewer(s) for their contribution to the peer reivew of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–18 containing materials and methods, procedures, crystallographic data, further computational analysis, peptide labelling studies, evaluation of ADME properties, unsuccessful reactions, coordinates and spectra data.
Supplementary Data (CIF)
Crystallographic data for 6s, 7f, 7h, 8d and 12. Deposited with the CCDC (CCDC 2175923–2175926 and CCDC 2176171).
Supplementary Data (Coordinates)
Folder containing all necessary .xyz files of computational analysis. Deposited in the Oxford Research Archive.
Supplementary Data (source data)
Physicochemical and pharmacokinetic property data. Deposited in the Oxford Research Archive.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frank, N., Nugent, J., Shire, B.R. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022). https://doi.org/10.1038/s41586-022-05290-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05290-z
This article is cited by
-
C−F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres
Nature Communications (2024)
-
2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
Nature Chemistry (2023)
-
General access to cubanes as benzene bioisosteres
Nature (2023)
-
2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
Nature Communications (2023)
-
Hydrogen-bond-acceptor ligands enable distal C(sp3)–H arylation of free alcohols
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.