Abstract
Posttraumatic stress disorder (PTSD) is associated with altered pain perception, namely increased pain threshold and higher pain response. While pain consists of physiological and affective components, affective components are often overlooked. Similar patterns of increased threshold-high response in PTSD were shown in response to emotional stimuli, i.e., emotional numbing. As both emotional numbing and pain processing are modulated by the amygdala, we aimed to examine whether individuals diagnosed with PTSD show lower amygdala activation to pain compared with combat controls, and whether the amygdala responses to pain correlates with emotional numbing. To do so, two independent samples of veterans (original study: 44 total (20 PTSD); conceptual replication study: 40 total (20 PTSD)) underwent threat conditioning, where a conditioned stimulus (CS+; visual stimulus) was paired with an unconditioned stimulus (US; electric-shock). We contrasted the amygdala activity to the CS + US pairing with the CS+ presented alone and correlated it with emotional numbing severity. In both samples, the PTSD group showed a robust reduction in amygdala reactivity to shock compared to the Combat Controls group. Furthermore, amygdala activation was negatively correlated with emotional numbing severity. These patterns were unique to the amygdala, and did not appear in comparison to a control region, the insula, a pivotal region for the processing of pain. To conclude, amygdala response to pain is lower in individuals with PTSD, and is associated with emotional numbing symptoms. Lower amygdala reactivity to mild pain may contribute to the “all-or-none” reaction to stressful situations often observed in PTSD.
Similar content being viewed by others
Introduction
Neuroimaging studies of posttraumatic stress disorder (PTSD) have consistently shown exaggerated amygdala activation [1], both in response to trauma-related stimuli and generic emotional stimuli [2]. This is in line with the hypothesis that PTSD results from dysregulation of fear [3], in which initial fear from the traumatic event persists for months and years, a long-time after the trauma has passed. Consequently, Pavlovian fear conditioning is one of the most common behavioral paradigms used to study PTSD in humans [4,5,6]. In this paradigm, one stimulus (conditioned stimulus, CS+) is occasionally followed by an aversive unconditioned stimulus (US; e.g., electric shocks), and a second stimulus (CS−) is never followed by the aversive US. Pavlovian fear conditioning studies have typically shown increased skin conductance response (SCR) to the CS− (i.e., overgeneralization of fear) and prolonged extinction of the CS+ (i.e., inhibition of extinction) in PTSD, compared to non-PTSD populations [7]. Neuroimaging studies comparing PTSD patients and trauma-exposed controls further report increased amygdala and anterior hippocampus responses to the CS+, both during fear acquisition and late extinction phases [8]. In contrast, the neural response to the US (e.g., receiving a mild electric shock) is often overlooked.
Pain and PTSD are often tied together. The traumatic event that leads to the development of this debilitating disorder usually consists of actual pain or threat of pain [9], and not surprisingly there is a high comorbidity between PTSD and chronic pain disorders [10, 11]. Pain itself is often treated as a physiological phenomenon governed by the “pain matrix” [12], a subset of neural regions that are implicated in pain processing. While the exact composition of the matrix is still debated, the insula is the most consistently reported region [12]. The insula responds to sensory inputs and especially aversive ones, such as those used in fear conditioning studies [13, 14]. However, pain has an additional (often overlooked) affective aspect [15,16,17], largely modulated by amygdala functionality [18]. Indeed, individuals diagnosed with PTSD often show abnormalities in both the physical and affective processing of pain. While PTSD patients rate suprathreshold aversive stimuli as more painful, they also demonstrate higher sensation of pain threshold [19, 20], compared to healthy controls. This increased threshold for feeling pain can be blocked with opioids antagonists, such as naloxone and naltrexone [21,22,23], and is most often observed under stress, although it is not limited to stressful situations; thus it is referred to as “Stress-Induced Analgesia” [21]. Individuals with PTSD show greater pain suppression (i.e., higher stress-induced analgesia response) to acute pain compared to both healthy individuals [19] and trauma-exposed controls [22, 24], suggesting that stress-induced analgesia in PTSD is an exaggeration of a normal response (i.e., pain suppression under stress). The behavioral response characterizing stress-induced analgesia is commonly measured using self-reports [19, 22, 24]. However, there is scarce evidence of the amygdala response to pain during stressful or fear-inducing situations in PTSD.
The “high threshold-high response” to pain implicated in PTSD is similar to the response pattern to affective stimuli known as emotional numbing. Emotional numbing encompasses the restricted capacity to experience positive and/or negative emotions, as well as hyper-responsivity to highly negative stimuli [25, 26]. Emotional numbing was previously associated with several pain symptoms in PTSD patients [27,28,29], including fear of pain, pain intensity, and pain disability [29]. In addition, higher pain tolerance and emotional numbing are two of the most prominent symptoms reported by veterans after deployment [28]. Therefore, it is possible that emotional numbing and stress-induced analgesia share a common mechanism.
To test this possibility, we examined the affective response to mild pain, rather than suprathreshold pain, in the amygdala and its modulation by emotional numbing symptoms, in trauma-exposed combat veterans with and without PTSD during fear conditioning. Note that we did not test fear acquisition (CS+ >CS−), which has been extensively studied (for meta analysis see [8]). We hypothesized that participants with PTSD would show lower amygdala activation to mild pain compared to trauma-exposed controls (i.e., higher pain threshold), and that decreased amygdala’s activation would be associated with greater severity of emotional numbing symptoms. To assess the robustness of our findings, we further examine these hypotheses in an independent group of participants that performed a fear conditioning analogous paradigm (e.g., fear generalization) [30].
Materials and method
Study 1 (original sample)
Participants and clinical assessment
Fifty right handed veterans with combat experience ranging from the Vietnam war to current conflicts were recruited from the VA hospital in West Haven, Connecticut, and provided informed consent (see Table 1). All participants underwent clinical screening using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) [31] and the Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) [32]. Based on the latter, n = 25 participants met PTSD diagnosis (i.e., “PTSD” group) and n = 25 did not meet PTSD diagnosis (i.e., “Combat Controls” group). For exclusion criteria, please refer to the supplementary methods. In addition, participants completed two self-report questionnaires: PTSD Checklist for DSM-5 (PCL-5) [33] and Beck Depression Inventory-II (BDI-II) [34]. Six participants were excluded from the final analysis due to high movement ratio, framewise displacement (FD) > 0.4 [35] (3 from the PTSD group and 1 from the combat controls) or equipment failures (2 from the PTSD group). The remaining 44 participants (20 PTSD) were included in the final analyses. The study was approved by the Institutional Review Boards of Yale University (1103008132) and the VA Connecticut Healthcare System (IHR003). All participants gave informed consent and received monetary compensation for their participation.
Measures and analyses
Emotional numbing
Symptoms of emotional numbing were assessed using items 12–14 of the PCL-5 (possible score 0–12), based on the 7 factor model of PTSD [36, 37].
Fear conditioning task [38]
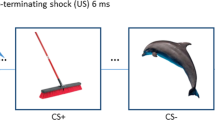
Participants were asked to observe three colored squares (blue, yellow, and green) presented on a screen and assess the relationship between these squares and the probability of receiving an electric shock. The order of appearance of the different stimuli was counterbalanced between participants to control for the order effect. Two of the colored squares (CS+) were each partially paired with shock, with 7 presentations of CS + US and 9 presentations of CS+ alone (for a total of 14 CS + US and 18 CS+, 43.75% reinforcement rate; see Fig. 1a). In addition, there were 9 presentations of the third square which was never paired with shock (safety signal, CS−). Squares appeared for 4 s (with an ITI of 6–10 s). In CS + US trials, the shock was applied for 200 ms trains and overlapped with the offset of the square.
A In study 1, participants watched a pseudo-random series of three colored squares. Two of these colored squares co-terminated with a 200 ms US (i.e., electric shock) in 14 out of the 32 (43.75%) presentations (each colored-squared was paired with shock 7 out of 16 times). A third-colored square appeared 9 times and was never paired with the US (shock). B In Study 2, adapted from Kaczkurkin et al. [30], participants saw a pseudo random order of checkerboard patterned rings or a “V” shaped object. Each stimulus was presented for 4 s with an ITI of 2.4–4.8 s. Only the CS+ co-terminated with a 100 ms US (i.e., electric shock) in 22 out of the 35 (63%) presentations. The study included rings of 5 sizes, with one serving as CS+ and the rest as CS−. The different sizes were used to test generalization in the original study.
Electric shock
The shock was administered by two electrodes placed on the inner wrist of the participant’s dominant hand, connected to a Constant Voltage Stimulator—Unipolar Pulse (Model STM200; Biopac Systems, Inc., Goleta, CA). Shock levels were personally tailored for each participant. Starting at a minimal shock level (20 volts), the shock intensity was gradually increased by the experimenter. Participants were asked to report when the shock was “highly unpleasant but not painful”, and this level was set for them throughout the entire duration of the experiment.
Skin conductance response (SCR)
Individuals’ physiological responses were assessed using two Ag–AgCl electrodes, connected to a BioPac Systems skin conductance module (EDA100C). The electrodes were attached to the first and second fingers of each participant’s non-dominant hand, between the first and second phalanges. SCR waveforms were analyzed offline, using LedaLab version 3.4.9 (www.ledalab.de). Physiological data was downsampled to 100 HZ and smoothed using a Gaussian window (size of 8 samples). Next, SCRs were decomposed by continuous decomposition analysis (CDA) [39], extracting the phasic information underlying the skin conductance response. Maximum phasic driver-peaks (mS > 0.02) in a time window of 0.5 to 4.5 s after shock onset were extracted.
Magnetic resonance imaging (MRI)
MRI data were collected using a 3 T Siemens Prisma scanner at the Yale Magnetic Resonance Research Center (MRRC), using a 32-channel receiver array head coil. High-resolution structural images were acquired by Magnetization-Prepared Rapid Gradient-Echo (MPRAGE) imaging (TR = 2.5 s, TE = 2.83 ms, FOV = 256 × 256 mm2, matrix = 256 x 256 mm2, slice thickness = 1.0 mm without gap, 160 slices, voxel size 1.0 × 1.0 × 1.0 mm3). Functional MRI scans were acquired during the fear conditioning task, using a multi-band (4) Echo-planar Imaging (EPI) sequence (TR = 1000 ms, TE = 30 ms, flip angle = 60°, voxel size = 2 × 2 × 2 mm3, 60 2 mm-thick slices, in-plane resolution = 2 × 2 mm2, FOV = 220 mm).
Neural data preprocessing
All preprocessing stages were performed using fMRIPrep 20.0.6 [40] and following standard procedures (For neuroimaging acquisition and preprocessing details, see the Supplementary Methods).
Neural data analysis
All analyses were carried out using FSL imaging suite (version 6.00) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki), with double-gamma HRF, using FSL via the nipype interface [41, 42]. Each subject’s BOLD signal was smoothed using a 6-mm3 full width at half maximum (FWHM) Gaussian kernel. The general linear model (GLM) included the predictors for each condition (CSa+, CSa+US, CSb+, CSb+US, CSa−, CSb−), to correct for nuisance we have also added the following covariate of non-interest: effects of motion estimated during the realignment step (total of 6 confounds -rotation and translation, framewise displacement (FD), spatial distortion (std DVARS), and noise (the first 6 anatomical components from CompCor) [43]. Amygdala/insula activation was examined in the contrasts: (1) CS + US > CS+ and (2) CS+> baseline between the two study groups (PTSD vs. Combat Controls); time assumed 4 s.
Study 2 (conceptual replication sample)
Participants and clinical assessment
Seventy-one veterans of the conflicts in Iraq and Afghanistan were recruited for the previously reported study by Kaczkurkin et al. [30]. All participants (N = 71) were screened using CAPS-IV [32], and based on that categorized into three groups: “PTSD” (N = 26), “Subthreshold PTSD” (CAPS score: 20–39; N = 23), and “Combat Controls” (CAPS score: 0 to 19; N = 22). As this sample was used for conceptual replication of the original study, the subthreshold group was excluded from the current analysis (see Table 1). In addition to the CAPS-IV, participants completed the self-report Posttraumatic Stress Disorder Checklist—Military Version (PCL-M) [44]. Those who did not show fear response (4 PTSD; 1 combat control) and those with excessive head motion (>3 mm in any direction between consecutive EPI volumes; 2 PTSD; 1 combat control) were excluded from the final analysis, resulting in a final sample of 40 participants in the final analysis (20 PTSD). For more details, see Kaczkurkin et al. [30].
Emotional numbing
Kaczkurkin et al. [30] collected PCL-M, unlike PCL-5 in the original sample, hence, emotional numbing was assessed using items 8–12 (possible score 5–25), based on the 5-factor model of PTSD [45].
Fear conditioning (generalization) task [30]
Participants were asked to view stimuli and were instructed that they “might learn to predict the shock if they attend to the presented stimuli”. The original CS+ was a checkerboard textured ring (see Fig. 1b). For the generalizability task, there were 5 sizes for the ring, with only one size (biggest/smallest; counterbalanced) associated with a shock. Two different safety cues were used for the CS− (ring-shaped biggest/smallest or ‘V’-shaped stimuli). The CS+ was presented 35 times, with 22 co-terminated with a shock (CS + US; 63% reinforcement rate). The US was a 100 ms electric shock (3–5 mA individually adjusted to a “highly uncomfortable or mildly painful” level) delivered to the right ankle.
Preprocessing conceptual replication
All preprocessing was performed using Analysis of Functional Neural Images (AFNI) [46] (for complete details, see Kaczkurkin et al. [30] and the Supplementary materials). Both datasets were analyzed using the same ROI analysis and robust regression analysis (see study 1 methods).
General methods
Region-of-Interest (ROI) analysis
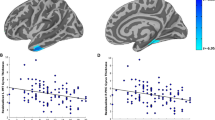
To examine the amygdala’s response to pain, we conducted an ROI analysis. To assess the specific role of the amygdala in affective processing of pain, the insula, an area associated with the physical properties of pain, was used for comparison [12]. Bilateral amygdala and insula ROI masks were taken from the “Neurosynth” database [47] using the terms “amygdala” and “insula”, respectively (see Fig. 2a, b). Amygdala activation, across both hemispheres and all voxels included in the mask, was averaged to a single amygdala activation score per subject. Amygdala’s activation analysis between groups (PTSD vs. Combat Controls) was conducted using Stan statistical language, via a cmdStanpy interface. The same steps were repeated for the insula.
Statistical analysis
All analyses were conducted in Stan, a probabilistic programming language, using its interface with Python (cmdStanPy). All results are reported based on the posterior distribution using mean and 89% Highest Posterior Density Interval (HPDi) [48, 49]. Comparison of amygdala activation between groups was done using a simple linear model, with amygdala’s average activation as the dependent variable and group (PTSD/Combat Controls) as the independent variable. Partially informed priors were used in these analyses, with both slope and intercept assumed to be normally distributed (Mean = 0,SD = 1). Emotional numbing served as the dependent variable and amygdala activity to shock was the independent variable with the intercept and coefficient prior normally distributed (Mean = 0, SD = 10, as the PCL scores have higher variance) [50].
Robust Bayesian regression analysis
To examine the association between amygdala and insula response to mild pain (i.e., electric shock) and emotional numbing symptoms, and to reduce the influence of outliers on the model, a robust Bayesian regression analysis was conducted [51]. Participants’ emotional numbing score was set as the dependent variable with amygdala or insula average activation as the independent variable. Partially informed priors were used in these analyses, with the intercept and slope prior normally distributed (Mean=0, SD = 10, as PCL scores have higher variance) [50]. For the independent variable (i.e., amygdala or insula), the model used a Student’s t distribution (to account for outliers) with the ν prior distributed as a Gamma distribution (k = 2, θ = 0.1) as prior [49, 52, 53]. All Stan models can be found in the study GitHub repository (https://github.com/LevyDecisionNeuroLab/SIA_PTSD).
Results
Demographic and clinical characteristics
Demographics and clinical characteristics of the original and conceptual replication samples are presented in Table 1. No significant differences were found between PTSD and Combat Controls in age, SCR response to the US, or the personally selected shock levels between the group (p > 0.05; SCR data available only for study 1).
Decreased amygdala responsivity to shock in PTSD patients
Consistent with our hypothesis, the PTSD group showed decreased bilateral amygdala activation to the shock compared to the Combat Controls group (Study 1: β = −0.20; SD = 0.11; 89% HPDi = [−0.40, −0.02]; Fig. 3a; Study 2: β = −0.16; SD = 0.08; 89% HPDi = [−0.29, −0.02]; Fig. 3b). There was no group difference in the amygdala response to the non-reinforced CS+ stimuli (i.e., vs. fixation; Study 1: β = 0.073; SD = 0.20, 89% HPDi = [−0.29, 0.40]; Study 2: β = −0.031; SD = 0.05, 89% HPDi = [−0.12, 0.05]), suggesting that the difference is specific to the shock administration, and does not result from overall heightened arousal in the PTSD group.
Average neural activation to shock (i.e., the contrast of paired CS + US vs. unpaired CS+ trials) in the Combat Control (blue) and PTSD (orange) groups. The right side of each figure depicts the curve of the resampled distribution of differences between the two groups (PTSD - CC). The mean of the PTSD group relative to controls is indicated by the black dot, and the 89% confidence interval is indicated by the thick black line. Results from the original sample (Study 1) are presented in A, C, while results from the conceptual replication sample (Study 2) are presented in B, D. Results of the bilateral amygdala are presented in A, B, whereas results of the bilateral insula are presented in C, D.
To assess the specificity of the effect to the amygdala, we tested the same hypothesis in a different neural region (bilateral Insula, see methods). As expected, in both the original and replication cohorts, there was no group difference in bilateral Insula activation to the shock (Study 1: β = −0.01; SD = 1.4; 89% HPDi = [−0.35, 0.12]; Study 2: β = −0.018; SD = 0.06; 89% HPDi = [−0.12, 0.08]).
Amygdala’s responsivity to shock is associated with emotional numbing symptoms
Consistent with our hypothesis, Robust regression analysis revealed a negative correlation between amygdala activation and the emotional numbing score (normalized emotional numbing scores: Study 1: ϱ = −0.3, SD = 0.1, 89% HPDi = [−0.5, −0.12]; Fig. 4a; Study 2: ϱ = −0.2, SD = 0.1, 89% HPDi = [−0.38, −0.02]; Fig. 4b), such that lower amygdala reactivity to the shock corresponded to higher emotional numbing scores.
Robust Bayesian regression between average neural activation to shock (i.e., the contrast of paired CS + US vs. unpaired CS+ trials) and emotional numbing based on the PCL questionnaire (Study 1: PCL-5 items 12–14; Study 2: PCL-M items 8–12). Results from the original sample (Study 1) are presented in A, C, while results from the replication sample (Study 2) are presented in B, D. Results of the bilateral amygdala are presented in A, B, whereas results of the bilateral insula are presented in C, D.
In the specificity analysis, as expected, no significant association was found between the insula activation and emotional numbing (study 1: ϱ = −1.3, SD = 1.3, 89% HPDi = [−3.6, 0.9]; study 2: ϱ = −1.2, SD = 3.7, 89% HPDi = [−7.8, 4.9]).
Several control analyses were conducted on data from the original sample to assess the specificity of the suggested EN-amygdala link (see supplementary results for complete statistics). First, amygdala response was not correlated with depression symptoms score (BDI; ϱ = −3.9,SD = 5.3, 89% HPDi = [−13.0, 4.9]). Second, multiple robust regressions, each including emotional numbing and one other PTSD cluster from the 7 factor model of PTSD [36, 54], showed no robust additive predictive value for any other cluster (Avoidance: ϱ = 0.01, 89% HPDi = [−0.05, 0.07]; Intrusion: ϱ = 0.01, 89% HPDi = [−0.02, 0.04]; Negative affect: ϱ = −0.01, 89% HPDi = [−0.05, 0.02]; Externalized behavior: ϱ = 0.04, 89% HPDi = [−0.03, 0.11]; Dysphoric arousal: ϱ = 0.01, 89% HPDi = [−0.05, 0.07]; Anxious arousal: ϱ = 0.01, 89% HPDi = [−0.03, 0.06]). Finally, the results were not affected by movement artifacts: a group by condition analysis on framewise displacement (FD) showed no group (β = 0.01, SD = 0.02, 89% CI [−0.03, 0.04]), condition (β = −0, SD = 0.01, 89% CI[−0.02, 0.00]) or interaction effects (β = 0.01, SD = 0.01, 89% CI[−0.01, 0.03]).
Discussion
This study aimed to investigate affective neural processing of mild subthreshold pain and its relation to emotional numbing in individuals diagnosed with PTSD. To this end, we isolated the response of the amygdala and insula to electric shocks, an unconditioned stimuli during the acquisition stage of a fear conditioning task, in trauma-exposed combat veterans with and without PTSD diagnosis (PTSD and Combat Control group, respectively). To examine the replicability and generalizability of the results, we re-run the analysis using an independent sample. In both samples, we found an overall reduction in amygdala (but not insula) responsivity to mild pain in the PTSD group, compared with Combat Controls. Furthermore, amygdala (but not insula) responsivity was negatively correlated with the degree of emotional numbing symptoms, consistent with the suggested link between numbing of both emotions and emotional pain processing within the amygdala.
Results of this work provide indirect evidence for a common mechanism for emotional numbing and stress-induced analgesia. Foa and colleagues [48] were the first to suggest this “common-mechanism”, based on animal research of inescapable shock. Further evidence for this link between stress-induced analgesia and emotional numbing comes from studies showing that emotional numbing is correlated with: (1) increased pain symptoms in PTSD patients [29], (2) reduced functioning in chronic pain patients [55], and (3) increased pain disability in healthy individuals after surgery [56]. Moreover, emotional numbing may actually be context-dependent [25], expressed mostly in stressful situations (i.e., stress-induced numbing). Our findings further suggest that the potential shared mechanism for stress-induced analgesia and emotional numbing is regulated by amygdala activity (and not by the insula, a physical pain perception area), although causal manipulations are still needed to confirm this hypothesis. Indeed, several neurofeedback studies have shown that reducing amygdala response to threatening stimuli can reduce pain perception [57] and increase emotion regulation [58, 59].
Previous studies investigating the association between the amygdala response to mild pain in PTSD populations report inconsistent results, with some showing reduced activation to pain and others demonstrating the opposite. For example, Geuze and colleagues [60], using a block-design study, have shown reduced activation of the amygdala to heat pain in veterans with PTSD (compared to veterans without PTSD). However, their design used the interval between blocks as baseline, and thus does not account for the effects of anticipation on the amygdala [61]. In contrast, Linnman and colleagues [62], showed an increase in amygdala activity in PTSD compared with trauma control, to pain induction by electric shocks during a fear conditioning paradigm. In an attempt to control for anticipatory effects, they focused on the shock onset, as it coincided with the cue offset, thus, comparing the interval between trials. Nevertheless, in such a design, the brief shocks (0.5 s) account for a very small percentage of the recording time (TR = 3 s). Thus, the analyses focused mostly on blank screens, which can include unrelated noise as during resting-state scans [63]. Nevertheless, this period might also hold the positive prediction error, and thus the increase might represent value updating.
These contradictory results could be due to four main factors: pain type (heat vs. shock), pain levels selection (constant, self-tailored), paradigm (block-design vs. event-related fear conditioning paradigm), and recording window (few long continuous trials vs. many short windows). In our study, we focused on the entire period of the cue and the pain (CS + US). This window enabled us to control for the amygdala anticipatory response to the adverse event [64] while looking at the response to pain [65] with the introduction of as little noise as possible. We also used a recording sequence of TR = 1 s, which allowed better temporal resolution compared to previous studies. Our study used the more common, self tailored approach [66]. Thus, it increases the feeling of controllability and preparedness in the participant. In turn, it might initiate the analgesic response earlier. As recent work showing the difficulty to replicate results in fMRI studies, even when using the same exact dataset [67], our ability to replicate the results using an independent data set from a different group using an analogous paradigm [30] further strengthens the finding that PTSD patients have a diminished response in the amygdala to mild pain [20].
The most probable mechanism at the core of the relation between stress-induced analgesia and emotional numbing is μ-opioid receptor inhibition of the amygdala. During stress, the body secretes endorphins (endo-opioids) that reduce the sensation of pain, so the organism can better cope with a potential threat [68]. Both pain and affect trigger the release of endorphins in the amygdala [69, 70], which in turn, mediates the antinociceptive response [69]. Thus, higher endorphin-mediated inhibition of the amygdala response to mild stimuli propagates a lower amygdala response. In turn, a lower amygdala response fails to trigger an “appropriate” emotional response. This inhibition of pain and affect is supposed to help the organism cope with an immediate threat. However, in PTSD, where trauma reminders are constant, such lower emotional tone might cause emotional numbing. Alterations in the opioid system in individuals diagnosed with PTSD provide further support for this suggested theory. Indeed, PTSD was previously associated with at-rest lower plasma-endorphin tone [71], similar to chronic pain patients [72]. However in PTSD there is a steep incline in endorphins following stress [73]. Moreover, compared with trauma controls, individuals with PTSD show a higher binding potential of μ-opioid receptors in the amygdala [74]. These results explain how Naloxone, a μ-opioid antagonist, can block the effect of stress-induced analgesia [22, 75]. This pathophysiological mechanism might be at the core of many symptoms and deficiencies related to PTSD, such as impaired emotion regulation [76]. For example, by not initiating an appropriate emotional response to a stimulus in time, the individual might be less able to engage in effective emotion regulation strategies [77].
While our results are robust, several limitations should be noted. First, both samples included only veterans and were mainly males (Study 1: 90.1%; Study 2: 100%), which suggests a relatively homogeneous trauma type. Thus, we are limited in our ability to generalize our findings to other trauma types or females. In addition, all participants experienced trauma. Hence, future research should try and look at different trauma types, as well as no trauma and sex differences. Second, both studies used electrical shock to inflict pain. While our results support findings from Geuze et al., [60] who used heat pain, a more thorough view on the methods for inflicting pain and selecting threshold is needed. Third, our cross-sectional design cannot assess stability over time or directionality of the pain-EN relation. Future longitudinal studies may shed light on the causality of this relation. Finally, as our aim was only to look at the amygdala response to mild (sub-threshold) pain, we did not directly test pain thresholds or tolerance. Our results raise an important question, if indeed, the amygdala also mediates the higher pain response to suprathreshold stimuli. Future studies might be able to shed light on the amygdala response to painful stimuli from subthreshold to suprathreshold.
In conclusion, decreased amygdala activation to pain is linked to difficulty experiencing emotions (i.e., EN) in two independent samples of combat-exposed veterans. These findings further advance our understanding of the neural mechanisms underlying pain perception in PTSD and their relationship to an extensive literature investigating emotional numbing in PTSD. Future work is needed to test the hypothesis that opioid receptor inhibition of the amygdala contributes to the relationship between pain suppression and emotional numbing in PTSD.
Clinical implications from this research suggest that psychological treatment should aim at assisting PTSD patients to be more mindful of their feelings, especially in stressful situations, to be able to react to painful/emotional stimuli earlier and thus more effectively.
References
Koenigs M, Grafman J. Posttraumatic Stress Disorder: The Role of Medial Prefrontal Cortex and Amygdala. Neuroscientist. 2009;15:540–8.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87.
Kredlow AM, Fenster RJ, Laurent ES, Ressler KJ, Phelps EA. Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology. 2022;47:247–59.
Lissek S, van Meurs B. Learning Models of PTSD: Theoretical Accounts and Psychobiological Evidence. Int J Psychophysiol J Int Organ Psychophysiol. 2015;98:594–605.
Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35.
Dunsmoor JE, Cisler JM, Fonzo GA, Creech SK, Nemeroff CB. Laboratory models of post-traumatic stress disorder: The elusive bridge to translation. Neuron. 2022. https://doi.org/10.1016/j.neuron.2022.03.001.
Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. Updated Meta-Analysis of Classical Fear Conditioning in the Anxiety Disorders. Depress Anxiety. 2015;32:239–53.
Suarez-Jimenez B, Albajes-Eizagirre A, Lazarov A, Zhu X, Harrison BJ, Radua J, et al. Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: a meta-analysis of functional magnetic resonance imaging studies. Psychol Med. 2020;50:1442–51.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
Shipherd JC, Keyes M, Jovanovic T, Ready DJ, Baltzell D, Worley V, et al. Veterans seeking treatment for posttraumatic stress disorder: what about comorbid chronic pain? J Rehabil Res Dev. 2007;44:153–66.
Scioli-Salter ER, Forman DE, Otis JD, Gregor K, Valovski I, Rasmusson AM. The Shared Neuroanatomy and Neurobiology of Comorbid Chronic Pain and PTSD: Therapeutic Implications. Clin J Pain. 2015;31:363–74.
Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: A coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehav Rev. 2016;68:120–33.
Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human Fear Conditioning and Extinction in Neuroimaging: A Systematic Review. PLOS ONE. 2009;4:e5865.
Labrenz F, Icenhour A, Schlamann M, Forsting M, Bingel U, Elsenbruch S. From Pavlov to pain: How predictability affects the anticipation and processing of visceral pain in a fear conditioning paradigm. NeuroImage 2016;130:104–14.
Pace MC, Mazzariello L, Passavanti MB, Sansone P, Barbarisi M, Aurilio C. Neurobiology of pain. J Cell Physiol. 2006;209:8–12.
Neugebauer V. Amygdala Pain Mechanisms. In: Schaible H-G, editor. Pain Control, Berlin, Heidelberg: Springer; 2015. p. 261–84.
Peirs C, Seal RP. Neural circuits for pain: Recent advances and current views. Science. 2016;354:578–84.
Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E. Porreca F. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology. 2020;170:108052.
Defrin R, Schreiber S, Ginzburg K. Paradoxical Pain Perception in Posttraumatic Stress Disorder: The Unique Role of Anxiety and Dissociation. J Pain. 2015;16:961–70.
Defrin R, Ginzburg K, Solomon Z, Polad E, Bloch M, Govezensky M, et al. Quantitative testing of pain perception in subjects with PTSD – Implications for the mechanism of the coexistence between PTSD and chronic pain. PAIN 2008;138:450–9.
Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202.
van der Kolk BA, Greenberg MS, Orr SP, Pitman RK. Endogenous opioids, stress induced analgesia, and posttraumatic stress disorder. Psychopharmacol Bull. 1989;25:417–21.
Ford GK, Finn DP. Clinical correlates of stress-induced analgesia: Evidence from pharmacological studies. PAIN. 2008;140:3–7.
Diener SJ, Wessa M, Ridder S, Lang S, Diers M, Steil R, et al. Enhanced stress analgesia to a cognitively demanding task in patients with posttraumatic stress disorder. J Affect Disord. 2012;136:1247–51.
Litz BT, Gray MJ, Gray MJ. Emotional Numbing in Posttraumatic Stress Disorder: Current and Future Research Directions. Aust N. Z J Psychiatry. 2002;36:198–204.
Sippel LM, Watkins LE, Pietrzak RH, Hoff R, Harpaz-Rotem I. The Unique Roles of Emotional Numbing and Arousal Symptoms in Relation to Social Connectedness Among Military Veterans in Residential Treatment for PTSD. Psychiatry. 2018;81:271–82.
Langford DJ, Theodore BR, Balsiger D, Tran C, Doorenbos AZ, Tauben DJ, et al. Number and Type of Post-Traumatic Stress Disorder Symptom Domains Are Associated With Patient-Reported Outcomes in Patients With Chronic Pain. J Pain. 2018;19:506–14.
Lusk J, Brenner LA, Betthauser LM, Terrio H, Scher AI, Schwab K, et al. A Qualitative Study of Potential Suicide Risk Factors Among Operation Iraqi Freedom/Operation Enduring Freedom Soldiers Returning to the Continental United States (CONUS). J Clin Psychol. 2015;71:843–55.
López-Martínez AE, Ramírez-Maestre C, Esteve R. An examination of the structural link between post-traumatic stress symptoms and chronic pain in the framework of fear-avoidance models. Eur J Pain. 2014;18:1129–38.
Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, et al. Neural Substrates of Overgeneralized Conditioned Fear in PTSD. Am J Psychiatry. 2016;174:125–34.
First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In: Hilsenroth MJ, Segal DL, editors. Comprehensive handbook of psychological assessment, Vol. 2. Personality assessment. John Wiley & Sons, Inc.; 2004. p. 134–143.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90.
Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383.
Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996.
Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536–51.
Duek O, Spiller TR, Pietrzak RH, Fried EI, Harpaz‐Rotem I. Network analysis of PTSD and depressive symptoms in 158,139 treatment‐seeking veterans with PTSD. Depress Anxiety. 2021;38:554–62.
Pietrzak RH, Tsai J, Armour C, Mota N, Harpaz-Rotem I, Southwick SM. Functional significance of a novel 7-factor model of DSM-5 PTSD symptoms: results from the National Health and Resilience in Veterans study. J Affect Disord. 2015;174:522–6.
Schiller D, Kanen JW, LeDoux JE, Monfils M-H, Phelps EA. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci. 2013;110:20040–5.
Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods. 2010;190:80–91.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. FMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219.
Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinformatics. 2011;5:13.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101.
Weathers FW, Huska JA, Keane TM. The PTSD Checklist Military Version (PCL-M). National Center for Posttraumatic Stress Disorder, Boston, MA; 1991. p. 1.
Elhai JD, Biehn TL, Armour C, Klopper JJ, Frueh BC, Palmieri PA. Evidence for a unique PTSD construct represented by PTSD’s D1–D3 symptoms. J Anxiety Disord. 2011;25:340–5.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
McElreath R. rethinking: Statistical Rethinking book package. R package version. 2014;1:391.
McElreath R. Statistical rethinking: A Bayesian course with examples in R and Stan. Chapman and Hall/CRC; 2018. p. 87–88.
Juárez MA, Steel MFJ. Model-Based Clustering of Non-Gaussian Panel Data Based on Skew-t Distributions. J Bus Econ Stat. 2010;28:52–66.
Berger JO, Moreno E, Pericchi LR, Bayarri MJ, Bernardo JM, Cano JA, et al. An overview of robust Bayesian analysis. Test. 1994;3:5–124.
Geweke J. Bayesian treatment of the independent Student‐t linear model. J Appl Econ. 1993;8:S19–S40.
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. Chapman and Hall/CRC; 2013. p. 435–449.
Claycomb Erwin M, Charak R, Durham TA, Armour C, Lv X, Southwick SM, et al. The 7-factor hybrid model of DSM-5 PTSD symptoms and alcohol consumption and consequences in a national sample of trauma-exposed veterans. J Anxiety Disord. 2017;51:14–21.
Clapp JD, Beck JG, Palyo SA, Grant DM. An examination of the synergy of pain and PTSD on quality of life: Additive or multiplicative effects? PAIN. 2008;138:301–9.
Katz J, Asmundson GJG, McRae K, Halket E. Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomy. Eur J Pain. 2009;13:870–8.
Goldway N, Ablin J, Lubin O, Zamir Y, Keynan JN, Or-Borichev A, et al. Volitional limbic neuromodulation exerts a beneficial clinical effect on Fibromyalgia. NeuroImage. 2019;186:758–70.
Herwig U, Lutz J, Scherpiet S, Scheerer H, Kohlberg J, Opialla S, et al. Training emotion regulation through real-time fMRI neurofeedback of amygdala activity. NeuroImage. 2019;184:687–96.
Zotev V, Phillips R, Young KD, Drevets WC, Bodurka J. Prefrontal Control of the Amygdala during Real-Time fMRI Neurofeedback Training of Emotion Regulation. PLOS ONE. 2013;8:e79184.
Geuze E, Westenberg HGM, Jochims A, Kloet CS, de, Bohus M, Vermetten E, et al. Altered Pain Processing in Veterans With Posttraumatic Stress Disorder. Arch Gen Psychiatry. 2007;64:76–85.
Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory Activation in the Amygdala and Anterior Cingulate in Generalized Anxiety Disorder and Prediction of Treatment Response. Am J Psychiatry. 2009;166:302–10.
Linnman C, Zeffiro TA, Pitman RK, Milad MR. An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord. 2011;1:1–12.
Finn ES. Is it time to put rest to rest? Trends Cogn Sci. 2021;25:1021–32.
Roozendaal B, McEwen BS. Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1:9.
Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, et al. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev. 2017;77:247–85.
Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, Johannesson M, et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–88.
Volpicelli J, Balaraman G, Hahn J, Wallace H, Bux D. The Role of Uncontrollable Trauma in the Development of PTSD and Alcohol Addiction. Alcohol Res Health. 1999;23:256–62.
Zubieta J-K, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional Mu Opioid Receptor Regulation of Sensory and Affective Dimensions of Pain. Science. 2001. https://doi.org/10.1126/science.1060952.
Zubieta J-K, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of Human Affective Responses by Anterior Cingulate and Limbic µ-Opioid Neurotransmission. Arch Gen Psychiatry. 2003;60:1145–53.
Hoffman L, Watson PB, Wilson G, Montgomery J. Low Plasma β-Endorphin in Post-Traumatic Stress Disorder. Aust N. Z J Psychiatry. 1989;23:269–73.
Almay BGL, Johansson F, Von Knorring L, Terenius L, Wahlstrom A. Endorphins in chronic pain. I. Differences in CSF endorphin levels between organic and psychogenic pain syndromes. Pain. 1978;5:153–62.
Hamner MB, Hitri A. Plasma beta-endorphin levels in post-traumatic stress disorder: A preliminary report on response to exercise-induced stress. J Neuropsychiatry Clin Neurosci. 1992;4:59–63.
Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, et al. Altered Central μ-Opioid Receptor Binding After Psychological Trauma. Biol Psychiatry. 2007;61:1030–8.
al’Absi M, Nakajima M, Bruehl S. Stress and pain: modality-specific opioid mediation of stress-induced analgesia. J Neural Transm. 2021;128:1397–407.
Frewen PA, Lanius RA. Toward a Psychobiology of Posttraumatic Self-Dysregulation. Ann N. Y Acad Sci. 2006;1071:110–24.
Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2:271.
Funding
This work was supported by NIH R01MH105535 to DS and IHR, CSRD CX001538 grant to IHR, NIH R01MH118215 to IL, and NSF BCS-1829439 to IL. DS is supported by the NIMH (R01MH122611, R01MH123069) and the Misophonia Research Fund. Replication study was originally supported by NIMH grant MH-080130 to SL.
Author information
Authors and Affiliations
Contributions
NK conceptualization and design, data analysis, interpretation of the data, writing initial draft, editing manuscript, gave final approval for the version to be published and is accountable for all aspects of the work including accuracy and integrity. OD conceptualization and design, acquisition of data, data analysis, interpretation of the data, writing initial draft, editing manuscript, gave final approval for the version to be published. ZBZ interpretation of the data, editing manuscript, gave final approval for the version to be published. ANK acquisition of data, data analysis, editing manuscript, gave final approval for the version to be published. SL acquisition of data, editing manuscript, gave final approval for the version to be published. TO acquisition of data, data analysis, gave final approval for the version to be published. DS interpretation of the data, editing manuscript, gave final approval for the version to be published. IHR interpretation of the data, editing manuscript, gave final approval for the version to be published. IL interpretation of the data, editing manuscript, gave final approval for the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Korem, N., Duek, O., Ben-Zion, Z. et al. Emotional numbing in PTSD is associated with lower amygdala reactivity to pain. Neuropsychopharmacol. 47, 1913–1921 (2022). https://doi.org/10.1038/s41386-022-01405-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01405-2