Abstract
Adenoid ameloblastoma is a very rare benign epithelial odontogenic tumor characterized microscopically by epithelium resembling conventional ameloblastoma, with additional duct-like structures, epithelial whorls, and cribriform architecture. Dentinoid deposits, clusters of clear cells, and ghost-cell keratinization may also be present. These tumors do not harbor BRAF or KRAS mutations and their molecular basis appears distinct from conventional ameloblastoma but remains unknown. We assessed CTNNB1 (beta-catenin) exon 3 mutations in a cohort of 11 samples of adenoid ameloblastomas from 9 patients. Two of the 9 patients were female and 7 male and in 7/9 patients the tumors occurred in the maxilla. Tumors of 4 of these 9 patients harbored CTNNB1 mutations, specifically p.Ser33Cys, p.Gly34Arg, and p.Ser37Phe. Notably, for one patient 3 samples were analyzed including the primary tumour and two consecutive recurrences, and results were positive for the mutation in all three tumors. Therefore, 6/11 samples tested positive for the mutation. In the 6 mutation-positive samples, ghost cells were present in only 2/6, indicating beta-catenin mutations are not always revealed by ghost cell formation. Dentinoid matrix deposition was observed in 5/6 mutation-positive samples and clear cells in all 6 cases. None of the cases harbored either BRAF or KRAS mutations. Beta-catenin immunoexpression was assessed in the samples of 8 patients. Except for one wild-type case, all cases showed focal nuclear expression irrespective of the mutational status. Together with the absence of BRAF mutation, the detection of beta-catenin mutation in adenoid ameloblastomas supports its classification as a separate entity, and not as a subtype of ameloblastoma. The presence of this mutation may help in the diagnosis of challenging cases.
Similar content being viewed by others
Introduction
Adenoid ameloblastoma, also referred to as adenoid ameloblastoma with dentinoid, is a very rare epithelial odontogenic neoplasm. Adenoid ameloblastoma is locally infiltrative, with an aggressive clinical behavior and high recurrence rates after enucleation (approximately 70%)1,2,3. Approximately 40 cases have been published revealing a peak incidence in the 4th decade (range 25–52 years), slight female predominance, and similar demographics to ameloblastoma1,2. It tends to affect the mandible (64.7%) and it is usually characterized by a painless swelling2. Radiographically, at diagnosis the majority (~82%) of tumors have presented as radiolucent lesions, or with occasional radiopaque foci, ill-defined borders, and cortical perforation.
Histologically, adenoid ameloblastoma is characterized by the presence of epithelium resembling conventional ameloblastoma, with additional duct-like structures, epithelial whorls, and cribriform architecture1,2,3,4. Dentinoid deposits, clusters of clear cells, and ghost-cell keratinization may also be present1,2,4. Some of these features resemble ameloblastoma, and adenoid elements resemble adenomatoid odontogenic tumor1. On the basis of the microscopic similarities to ameloblastoma and adenomatoid odontogenic tumor, our group recently screened a convenience sample of adenoid ameloblastoma for BRAF p.Val600Glu and KRAS p.Gly12Val and p.Gly12Arg mutations4, which are hallmarks of ameloblastomas and adenomatoid odontogenic tumors, respectively5,6,7. All nine samples tested were wild-type for both these pathogenic mutations4.
Another histopathological differential diagnosis for adenoid ameloblastoma is dentinogenic ghost cell tumor, and aggressive cases may show overlapping microscopic features with odontogenic carcinoma with dentinoid, for which no clear distinguishing diagnostic criteria have been established1,2. Dentinogenic ghost cell tumors8,9, and odontogenic carcinoma with dentinoid10 harbor CTNNB1 (beta-catenin) exon 3 mutations, similar to other lesions rich in ghost cells such as calcifying odontogenic cysts11.
Given the absence in adenoid ameloblastoma of the signature mutations of adenomatoid odontogenic tumor and ameloblastoma and the presence of CTNNB1 mutation in other microscopic mimics of adenoid ameloblastomas, we assessed CTNNB1 gene mutations in adenoid ameloblastoma.
Materials and Methods
Ethical aspects
This study was approved by The Research Ethics Committee of Universidade Federal de Minas Gerais (protocol number CAAE/approval: 30556120.0.0000.5149/4.228.043) and followed the Declaration of Helsinki. A convenience sample of 16 formalin-fixed paraffin-embedded adenoid ameloblastoma from 14 adenoid ameloblastoma cases was obtained from oral pathology services from the authors’ institutions. From the initial convenience sample (n = 16), 5 cases could not be analyzed due to limited genomic DNA (gDNA) available or poor-quality chromatograms, leaving 11 samples from 9 cases for analysis. Three samples were derived from a single patient who developed 2 recurrent tumors with a 6 year-interval after surgical enucleation of the primary tumor. Hematoxylin-eosin-stained slides of all cases were examined following the criteria used by Loyola et al.1
DNA isolation and Sanger sequencing
gDNA was isolated from formalin-fixed paraffin-embedded (FFPE) samples using the QIAamp® DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. A spectrophotometer (Nano-DropTM 2000; Thermo Fisher Scientific, Wilmington, DE, USA) was used to evaluate both the DNA concentration and quality.
Samples were screened by Sanger sequencing for CTNNB1 exon 3 mutations reported previously in the so-called ghost cell lesions and odontogenic carcinoma with dentinoid, which include the residues Asp32, Ser33, Gly34, Ser37, Thr41, and Ser458,9,10,11. Other codons within the amplicon were also inspected for mutations. PCR was performed using MyTaq HS Red Mix, 2x (Bioline Reagents, London, UK). Primers were designed to amplify exon 3 of the CTNNB1 gene using Primer3 (accessed at https://primer3.ut.ee/). The designed primers were F: 5′TTTGATGGAGTTGGACATGG3′ and R: 5′CAGGACTTGGGAGGTATCCA3′. M13 tails were added to the primers in order to facilitate the workflow and data analysis. Positive and negative controls were included in all reactions. Four of the cases included in the current study (cases #1-4) have previously been shown to harbor wild-type sequences for KRAS and BRAF4 mutations (Table 1). We further evaluated such mutations in the remaining cases included herein by using Sanger sequencing. The primers were F: 5′GGCCTGCTGAAAATGACTGAA3′ and R: 5′GGTCCTGCACCAGTAATATGC3′ for KRAS; and F: 5′TCATAATGCTTGCTCTGATAGGA3′ and R: 5′CCAAAAATTTAATCAGTGGA3′ for the BRAF gene.
PCR products were analyzed by electrophoresis and purified using ExoSAP‐IT™ PCR Product Cleanup Reagent (Applied Biosystems, Foster City, CA, USA). Bidirectional DNA sequencing was performed using Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and run on an ABI3130 DNA Analyzer (Applied Biosystems). The chromatograms were manually inspected in the SnapGene Viewer software (v. 5.3.2, from GSL Biotech; available at https://snapgene.com) using the reference sequence NM_001904.4 (CTNNB1), NM_004985.5 (KRAS), and NM_001354609.2 (BRAF) for comparison.
Immunohistochemistry
As the nuclear expression of beta-catenin is a surrogate marker for CTNNB1 exon 3 mutations, we also assessed the immunoexpression of beta-catenin in the cohort of adenoid ameloblastoma cases. 4 μm-thick sections of the FFPE samples were stained immunohistochemically using standard procedures as described elsewhere10,12. Immunohistochemistry was performed in all cases but one. Due to the limited amount of tissue, it was not possible to include Case #1.
Results
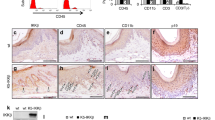
Microscopically, all samples showed epithelium resembling conventional ameloblastoma, duct-like spaces, and focal whorled cellular condensations reminiscent of morules (Fig. 1A–D), which are diagnostic criteria for this lesion. Clear cells were observed in all but one sample (Fig. 1B–D) and dentinoid matrix in 7/9 samples (Fig. 1E–G) (Table 1). Ghost cells (Fig. 1H) were observed in 4/9 cases (Table 1). Figure 1 shows the main histological findings. Table 1 summarizes the clinicopathological data and molecular status of each of the 11 samples from 9 patients included in the final analysis.
Cribriform arrangement of the ameloblastoma-like epithelial component, duct-like spaces, whirling or morules structures were observed in all cases (A–D). Clear-cell clusters (B–D) and dentinoid matrix deposits (E–G) were frequently observed. Ghost cells (H) were less often observed. Original magnification: A, C (10×); D, E, F (20×); B (30×); G, H (40×). Hematoxylin-eosin stains.
Six of the 11 samples tested positive for CTNNB1 mutation, including the 3 samples of the primary and recurrent tumor from patient #6 that showed concordant molecular results (Table 1). Therefore, tumors of 4 of the 9 patients (44%) harbored exon 3 CTNNB1 mutation, specifically at codons 33 (c.98C > G; p.Ser33Cys), 34 (c.100G > A; p.Gly34Arg) and 37 (c.110C > T; p.Ser37Phe). Chromatograms illustrating the variety of mutations identified and a summary of molecular and clinical data of the analysed samples are shown in Fig. 2. Notably, ghost cells were present in 2/6 samples positive for CTNNB1 mutations, and, conversely, in two cases with ghost cells a wild-type sequence was found (Table 1). There were no microscopic differences between wild-type and CTNNB1 mutation-positive cases. None of the cases harbored either BRAF (p.Val600Glu) or KRAS (codon 12) mutations (Table 1).
Samples of all but one case (7/8) showed focal positive nuclear and diffuse cytoplasmic immunoexpression of beta-catenin, irrespective of mutational status (Fig. 4, Table 1). Case #9 showed only cytoplasmic expression.
Discussion
Since its first description in the literature under a variety of names, adenoid ameloblastoma has become accepted as a rare pattern of odontogenic tumor showing histopathologic features resembling ameloblastoma and adenomatoid odontogenic tumor1,3. Although its status is unclear, adenoid ameloblastoma has usually been regarded as a rare variant of ameloblastoma, mainly due to its histopathologic similarities, aggressiveness, and high recurrence rates (~70%) with conservative treatment1,2,3. However, our research group recently assessed the presence of BRAF p.Val600Glu, signature mutations for ameloblastomas, and all tested adenoid ameloblastoma samples showed wild-type status4. Additionally, we screened these samples for KRAS mutations, which occur in 70% of adenomatoid odontogenic tumors6,7. None of the samples showed KRAS p.Gly12Val/Arg mutations4. Herein, we screened additional samples for these mutations, and all revealed wild-type sequences. Taken together, these results point to a different genetic background in adenoid ameloblastoma, ameloblastoma, and adenomatoid odontogenic tumor.
A recent study reported SMO and FGFR2 mutations in a single case of adenoid ameloblastoma13. These mutations have previously been reported in some ameloblastomas12,14, but not all features required for definitive diagnosis as adenoid ameloblastoma were present in this case making interpretation difficult. The incidence of SMO mutations in ameloblastoma ranges from 13 to 39%, occurring in a mutually exclusive pattern with BRAF p.V600E and co-occuring with additional RAS family or FGFR2 mutations12,14,15. Sweeney et al.14 proposed site-specific BRAF and SMO mutations in mandible and maxilla, respectively, which was later supported in a larger cohort15. However, such site-specificity for these mutations has not been confirmed by other groups16,17,18.
Compared to calcifying odontogenic cysts and dentinogenic ghost cell tumors, the diagnosis of adenoid ameloblastoma can be based on the presence of the pseudo-glandular arrangements, epithelial whorls, and the cribriform architecture1,3. Additionally, lower recurrence rates are achieved in calcifying odontogenic cysts upon conservative treatment1,19. Regarding ghost cell odontogenic carcinoma, more abundant ghost cells along with the malignant phenotype exhibited by neoplastic epithelium differentiate adenoid ameloblastoma19.
Odontogenic carcinoma with dentinoid is a further poorly characterized odontogenic tumour with some microscopic overlap with adenoid ameloblastoma. It is a rare malignant, low-grade, odontogenic neoplasm that is histopathologically characterized by the presence of cords and sheets of eosinophilic, pale, or clear epithelial cells associated with dentinoid material and, less commonly, duct-like structures10,20. Variable atypia and perineural invasion are occasionally reported10. The lack of, or minimal epithelium resembling conventional ameloblastoma distinguish it from adenoid ameloblastoma20. Importantly, pathogenic mutations in CTNNB1 and APC genes, components of the Wnt-signaling pathway, have been reported in this tumor10.
It is interesting to note that duct-like structures were previously reported in odontogenic carcinoma with dentinoid10. Despite the variable degrees of pleomorphism and high proliferative index observed in odontogenic carcinoma with dentinoid when compared with adenoid ameloblastoma, both exhibit some histopathological similarities, such as dentinoid material and the presence of clear cells. This finding, together with the fact that both share the same molecular driver, could suggest that they represent the benign and malignant counterparts of the same tumor. It is also interesting to note that the diagnosis of odontogenic carcinoma with dentinoid is based primarily on histologic features, particularly neural involvement by the tumor, but to date, no cases have been reported to metastasize. Additional studies are necessary to clarify any possible relationship between these entities.
The molecular basis and pathogenesis of adenoid ameloblastoma remain poorly explored. Dentinoid material and sometimes ghost cell keratinization are features shared with calcifying odontogenic cysts, dentinogenic ghost cell tumors, ghost cell odontogenic carcinoma, and odontogenic carcinoma with dentinoid, for which CTNNB1 mutations have core importance11,21,22. We screened adenoid ameloblastomas for these gene mutations and report CTNNB1 mutations in 4 of 9 (44%) cases of adenoid ameloblastoma.
CTNNB1 exon 3 mutations occurred at codons 33, 34, and 37. CTNNB1 encodes the beta-catenin protein, an important downstream effector of Wnt signaling, and has been associated with the oncogenesis of different neoplasms23. CTNNB1 exon 3 encodes the N-terminal domain of beta-catenin, where regulatory residues (Asp32, Ser33, Gly34, Ser37, Thr41, and Ser45) are located. Notably, most CTNNB1 exon 3 hotspot mutations culminate in alterations in these regulatory residues (Fig. 3)23,24. It is generally accepted that Ser45 residue is phosphorylated by casein kinase-1 alpha (CK-1α), priming it for glycogen synthase kinase 3 beta (GSK-3β) phosphorylation of Thr41, Ser33, and Ser37 residues. Asp32 and Gly34 residues are required for the interaction of beta-catenin with the ubiquitin E3 ligase beta-transducin repeats containing proteins (β-TrCP)23,24. In addition, Leu46 mutations may affect the phosphorylation efficiency by CK-1α25. Overall, CTNNB1 hotspot mutations disrupt the activity of the beta-catenin destruction complex, leading to beta-catenin nuclear and cytoplasmatic accumulation (Fig. 3).
The Wnt/beta-catenin signaling pathway regulates physiological processes including embryonic development, tissue homeostasis, and tissue regeneration. Disturbances in the Wnt/beta‐catenin pathway have been implicated as causes of several human neoplasms. We focused on the major pathway changes elicited by the wild-type and mutated CTNNB1 gene (upper and bottom panels, respectively), which encode for the beta-catenin protein. In the absence of Wnt ligands, cytoplasmic beta-catenin is phosphorylated by GSK-3beta and CK1α at N-terminal serine-threonine residues, leading to its destruction by the ubiquitin-proteasome pathway. Wnt binding to Frizzled and LRP cell-surface receptors prevents the phosphorylation-mediated degradation of the beta-catenin protein, thereby resulting in a significant increase in cytoplasmic levels of beta‐catenin. beta-catenin then translocates to the nucleus, where it can interact as a transcriptional coactivator with TCF/LEF, stimulating the expression of several nuclear targets. Hotspot mutations in the exon 3 (which encodes the N-terminal region of beta-catenin) affecting the phosphorylation/regulatory sites (amino acids Asp32, Ser33, Gly34, Ser37, Thr41, and Ser45) of the protein disrupts the phosphorylation-dependent ubiquitination (red arrows, bottom panel), then leading to beta-catenin accumulation and its protumorigenic effects. Exon 3 hotspot mutations of CTNNB1 are marked on the lollipop plot from cBioportal31,32.
Aberrant beta-catenin accumulation signals dysregulation of cell proliferation and metabolism, leading to tumorigenic effects23,24. In line with this, beta-catenin nuclear immunoexpression was observed in all the mutation-positive cases, and in 3/4 of the wild-type cases (Fig. 4). Considering that most of the wild-type cases also exhibited nuclear beta-catenin accumulation, mutations affecting other components of the beta-catenin destruction complex (e.g. inactivating mutations in APC or Axin tumor suppressor proteins) or alternative pathways’ crosstalk activating the pathway cannot be excluded8,10,26.
The 2017 WHO Classification of Head and Neck Tumors recognized three entities amongst the “ghost cell lesions family”: calcifying odontogenic cyst, dentinogenic ghost cell tumor, and ghost cell odontogenic carcinoma, all of which contain CTNNB1 mutations8,9,11,19. Additionally, ghost and clear cells are also observed in other tumors, including pilomatrixoma and adamantinomatous craniopharyngiomas, which also harbor CTNNB1 mutations together with additional genetic changes27,28,29. In the present study, CTNNB1 mutations were not restricted to cases containing ghost cells. Conversely, we did not detect mutations in some cases with ghost cells suggesting other changes may be required to develop this change.
Patient #6 of the present cohort had a primary tumor, originally diagnosed as adenomatoid odontogenic tumor, which was enucleated. The lesion recurred 6 years later and was considered a recurrence of adenomatoid odontogenic tumor. In the second recurrence, 6 years after the first recurrence, diagnosis was revised and the tumor was classified as adenoid ameloblastoma. Samples of the primary and recurrent tumors showed beta-catenin mutation p.Ser37Phe. The difficulties in reaching a final diagnosis illustrated by this case suggest that molecular assessment might be a helpful tool for challenging cases. As shown by our results, the presence of beta-catenin mutation would have favored the diagnosis of adenoid ameloblastoma, since such mutations do not occur in adenomatoid odontogenic tumors.
In summary, in the present study, we report for the first time the occurrence of CTNNB1 exon 3 mutations in adenoid ameloblastoma. The immunohistochemical nuclear expression of the beta-catenin suggests that this cellular pathway is activated in the tumor. This finding supports the new WHO classification of odontogenic tumours30 in classifying adenoid ameloblastoma as a separate entity from ameloblastoma and its subtypes but also raises the possibility of a relationship with odontogenic carcinoma with dentinoid and other ghost cell-containing odontogenic tumours.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Loyola AM, Cardoso SV, de Faria PR, Servato JPS, Eisenberg ALA, Dias FL, et al. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol. 120, 368–377 (2015).
Vered M, Wright JM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 16, 63–75 (2022).
Adorno-Farias D, Muniz VRVM, Soares AP, Cury PR, Rabelo RG, Fernández-Ramires R, et al. Ameloblastoma with adenoid features: A series of eight cases. Acta Histochem. 120, 468–476 (2018).
Coura BP, Santos JN, Fonseca FP, Bernardes VF, de Aquino SN, Júnior JJ, et al. Adenoid ameloblastoma with dentinoid is molecularly different from ameloblastomas and adenomatoid odontogenic tumors. J Oral Pathol Med. 50, 1067–1071 (2021).
Kurppa KJ, Catón J, Morgan PR, Ristimäki A, Ruhin B, Kellokoski J, et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol 232, 492–498 (2014).
Gomes CC, de Sousa SF, de Menezes GHF, Duarte AP, Pereira TdSF, Moreira RG, et al. Recurrent KRAS G12V pathogenic mutation in adenomatoid odontogenic tumours. Oral Oncol. 56, e3–e5 (2016).
Coura BP, Bernardes VF, de Sousa SF, França JA, Pereira NB, Pontes HAR, et al. KRAS mutations drive adenomatoid odontogenic tumor and are independent of clinicopathological features. Mod Pathol. 32, 799–806 (2019).
Kim S-A, Ahn S-G, Kim S-G, Park J-C, Lee S-H, Kim J, et al. Investigation of the β-catenin gene in a case of dentinogenic ghost cell tumor. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontol. 103, 97–101 (2007).
Rappaport MJ, Showell DL, Edenfield WJ. Metastatic ghost cell odontogenic carcinoma: description of a case and search for actionable targets. Rare Tumors 7, 96–97 (2015).
Gondak RO, Mariano FV, de Sousa SF, de Siqueira EC, Díaz KP, Martins LAL, et al. CTNNB1 and APC mutations in odontogenic carcinoma with dentinoid. Oral Surg Oral Med Oral Pathol Oral Radiol. 129, e249–e256 (2020).
Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, et al. β-Catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol. 163, 1707–1712 (2003).
Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 20, 5517–5526 (2014).
Jofre SA, Roth M, Lahouti AH, Gersten A, Azad AK, Kelsch RD, et al. Ameloblastoma with adenoid features: Case report with cyto‐histopathologic correlation and molecular findings. Diagn Cytopathol. 50, E140-E145 (2022).
Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA, et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 46, 722–725 (2014).
Gültekin SE, Aziz R, Heydt C, Sengüven B, Zöller J, Safi AF, et al. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch. 472, 807–814 (2018).
Diniz MG, Gomes CC, Guimarães BVA, Castro WH, Lacerda JCT, Cardoso SV, et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumor Biol. 36, 5649–5653 (2015).
Heikinheimo K, Huhtala J-M, Thiel A, Kurppa KJ, Heikinheimo H, Kovac M, et al. The Mutational Profile of Unicystic Ameloblastoma. J Dent Res. 98, 54–60 (2019).
Guimarães LM, Coura BP, Gomez RS, Gomes CC. The molecular pathology of odontogenic tumors: expanding the spectrum of MAPK pathway driven tumors. Front. Oral Health. 2, 740788 (2021).
El-Naggar A, Chan J, Grandis J, et al., eds. Odontogenic and maxilofacial bone tumours. In: WHO classification of Head and Neck Tumours, 4th ed. Lyon, France: IARC, 2017. p. 203–260.
Mosqueda-Taylor A, Neville BW, Tatemoto Y, Ogawa I, Takata T. Odontogenic carcinoma with dentinoid: a new odontogenic carcinoma. Head Neck Pathol. 8, 421–431(2014).
Yukimori A, Oikawa Y, Morita K-I, Nguyen CTK, Harada H, Yamaguchi S, et al. Genetic basis of calcifying cystic odontogenic tumors. PLoS One 12, e0180224 (2017).
de Sousa SF, Moreira RG, Gomez RS, Gomes CC. Interrogation of cancer hotspot mutations in 50 tumour suppressor genes and oncogenes in calcifying cystic odontogenic tumour. Oral Oncol. 57, e1–e3 (2016).
Kim S, Jeong S. Mutation hotspots in the β-Catenin gene: lessons from the human cancer genome databases. Mol Cells. 42, 8–16 (2019).
Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: A review. Oncotarget 9, 5492–5508 (2018).
Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, et al. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci USA. 100, 10193–10200 (2003).
Stamos JL, Weis WI. The β-Catenin Destruction Complex. Cold Spring Harb Perspect Biol. 5, a007898–a007898 (2013).
Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y, et al. Craniopharyngiomas of Adamantinomatous type harbor β-Catenin gene mutations. Am J Pathol. 161, 1997–2001 (2002).
Chan E, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 21, 410–413 (1999).
Gomes CC, de Sousa SF, Gomez RS. Craniopharyngiomas and odontogenic tumors mimic normal odontogenesis and share genetic mutations, histopathologic features, and molecular pathways activation. Oral Surg Oral Med Oral Pathol Oral Radiol. 127, 231–236 (2019).
Thavaraj S, Bilodeau EA. Adenoid Ameloblastoma. In: WHO Classification of Tumours Editorial Board. Head and neck tumours (WHO classification of tumours series, 5th ed.; vol. 9), 5th edn. Lyon (France): International Agency for Research on Cancer, 2022, [cited 2022 June 07]. Available from: https://tumourclassification.iarc.who.int/chapters/52.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 6, pl1 (2013).
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Acknowledgements
The authors acknowledge the Centro de Aquisição e Processamento de Imagens (CAPI- ICB/UFMG) for the technical support in image acquisition. The authors are thankful for the support of the Research Support Foundation of the State of Minas Gerais (FAPEMIG), the National Council for Scientific and Technological Development (CNPq), and the Coordination for the Improvement of Higher Education Personnel (CAPES). VCB and LMG receive a CAPES scholarship. BPC, RSG, PAV, and CCG are research fellows at CNPq.
Funding
The study was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [REDE 0019-16, PPM-00022-17] and the National Council for Scientific and Technological Development (CNPq), Brazil.
Author information
Authors and Affiliations
Contributions
CCG conceived the study and supervised the experiments. VCB, BPC, LMG, BGF, AC-LC, PAV, LB-R, LAM, JH, ST, JMW, EWO, RSG, and CCG contributed to sample and data acquisition and analysis. VCB, BPC, LMG, AC-LC, PAV, LB-R, LAM, JH, ST, JMW, EWO, RSG, and CCG worked on data interpretation. VCB, BPC, and CCG drafted the manuscript. All authors revised the article critically for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by Ethics Committee of Universidade Federal de Minas Gerais (UFMG, Brazil). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bastos, V.C., Coura, B.P., Guimarães, L.M. et al. Adenoid ameloblastoma harbors beta-catenin mutations. Mod Pathol 35, 1562–1569 (2022). https://doi.org/10.1038/s41379-022-01125-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01125-4
This article is cited by
-
Proceedings of the 2023 North American Society of Head and Neck Pathology Companion Meeting, New Orleans, LA, March 12, 2023: Odontogenic Tumors: Have We Achieved an Evidence-Based Classification
Head and Neck Pathology (2023)
-
Adenoid Ameloblastoma Versus Dentinogenic Ghost Cell Tumor
Head and Neck Pathology (2022)