Abstract
Ameloblastoma is a benign, locally aggressive odontogenic neoplasm with variable solid and cystic morphology. On account of its histologic variety, diagnostically challenging cases can bear resemblance to odontogenic keratocyst/keratocystic odontogenic tumor (KCOT) or dentigerous cyst (DC). BRAFV600E mutation has been reported to be specific for and frequent in ameloblastoma, and this study evaluated the usefulness of immunohistochemistry (IHC) using the BRAF VE1 mutant-specific antibody as a diagnostic adjunct in this setting. We investigated 46 ameloblastomas, 30 KCOTs, and 30 DCs. BRAF VE1 IHC was performed on all cases and allele-specific polymerase chain reaction (AS-PCR) for BRAFV600E mutation was performed on 30 ameloblastomas and any IHC-positive KCOT/DC. BRAF VE1 IHC was positive in 31/37 (83.8%) mandibular ameloblastomas but not in any maxillary ameloblastomas (0/9), KCOT (0/30), or DC (0/30). Equivocal staining was seen in 1/37 (3.3%) mandibular ameloblastomas. Of the 30 ameloblastomas subjected to AS-PCR, BRAFV600E mutation was identified in 19/23 (82.6%) mandibular ameloblastomas and 0/7 (0.0%) maxillary ameloblastomas. BRAFV600E mutant ameloblastomas were positive by IHC in 18/19 (94.7%) cases and equivocal in 1/19 (5.3%) cases. All 11 (100.0%) BRAF-wild type ameloblastomas were negative by IHC. BRAF VE1 is an excellent tool for the diagnosis of mandibular ameloblastoma but of limited utility in the maxilla, where it less commonly occurs and where BRAFV600E mutation is considerably less frequent.
Similar content being viewed by others
Introduction
Ameloblastoma is a benign, locally aggressive odontogenic neoplasm that conventionally exhibits variable solid and cystic architecture1. Growth as a single macrocystic structure is characteristic of unicystic ameloblastoma, a variant that may be associated with more indolent biologic behavior in certain instances2. On account of its frequent cystic presentation, the diagnosis of ameloblastoma can be challenging on biopsy specimens, particularly in light of the existence of numerous odontogenic cysts which, like ameloblastoma, may or may not be associated with an impacted tooth. Certain cystic entities, such as dentigerous cyst (DC) or odontogenic keratocyst/keratocystic odontogenic tumor (KCOT) are encountered more frequently than ameloblastoma in routine practice and, together with ameloblastoma, represent principal diagnostic considerations in the workup of a cystic mass from the jawbones.
Ameloblastomas characteristically present with prominent nuclear palisading, nuclear hyperchromasia, or reverse polarization of peripheral tumor cells, but these histopathologic features are often muted or lost in areas of cystic or plexiform growth. Additionally, KCOT exhibits nuclear palisading and hyperchromasia of basal cells, and the parakeratosis of KCOT can bear resemblance to the acanthomatous change seen in luminal tumor cells in ameloblastoma with cystic morphology. Correct diagnosis can usually be made on histopathologic features alone, even in biopsy specimens, but for challenging cases immunohistochemical adjuncts are lacking. Calretinin and CD56 expression has been reported in ameloblastoma, but has also been reported in KCOT to varying degrees, and these immunohistochemical stains are not widely used in clinical practice3,4,5,6.
Ameloblastoma is associated with MAPK pathway alterations in approximately 80–90% of cases, most commonly as a result of BRAFV600E mutation, and exhibits SMO mutations in approximately 15–40% of cases7,8,9. SMO mutations tend to co-occur with RAS or FGFR2 mutations, though rarely they may co-occur with BRAF mutations or may occur independently of MAPK pathway alterations, and it is unclear whether they represent a separate molecular subclass of ameloblastoma or a secondary genetic event8. Unicystic ameloblastoma has been found to harbor BRAFV600E mutation in over 90% of cases, and SMO mutations only rarely10. Other members of the ameloblastoma family of tumors, including ameloblastic fibroma, ameloblastic carcinoma and ameloblastic fibrosarcoma, are characterized by a similar mutational profile, which otherwise appears unique amongst odontogenic neoplasms or cysts11,12,13. KCOT, on the other hand, is characterized by a high prevalence of PTCH1 alterations in the absence of any SMO mutations or MAPK pathway signaling dysregulation14,15. DC is considered to represent reactive hyperplasia of dental follicular tissue in the setting of tooth impaction, in which the reduced enamel epithelium undergoes squamous metaplasia.
Several studies have shown BRAF VE1 immunohistochemistry (IHC) to have perfect or near-perfect concordance for molecular detection of BRAFV600E mutation in ameloblastoma, in keeping with the high sensitivity and specificity of this antibody for BRAFV600E mutation as demonstrated across multiple tumor types8,9,16,17,18,19. The aim of this study, therefore, was to evaluate the utility of BRAF VE1 IHC as an alternative marker of BRAFV600E mutation in the differential diagnosis of ameloblastoma and to determine its usefulness in this context.
Materials and Methods
Case selection
The study cohort consisted of 46 cases of ameloblastoma, 30 cases of KCOT and 30 cases of DC that were retrieved from the Department of Pathology, University Hospitals Cleveland Medical Center from 2000 to 2020. Hematoxylin and eosin (H&E) stained slides were reviewed for diagnostic confirmation by two pathologists (JW and IJS) based on 2017 WHO classification criteria. Following failed BRAF VE1 immunohistochemistry in five previously decalcified ameloblastoma specimens, the ameloblastoma cohort was restricted to biopsy specimens not previously subjected to decalcification. Thirty ameloblastoma specimens had adequate neoplastic cellularity (>30% tumor nuclei) for molecular testing; the remaining 16 specimens had insufficient tissue for molecular analysis on account of small sample size or inadequate neoplastic cellularity and were included to evaluate BRAF VE1 performance in the setting of limited neoplastic cellularity. All ameloblastoma cases included for molecular testing (cases 1–30) were in patients older than 18 years, in accordance with IRB approval. The study was approved by the Institutional Review Board of University Hospitals Cleveland Medical Center (STUDY20200628) and conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry
BRAF immunohistochemistry was performed on formalin-fixed, paraffin-embedded 4 µm whole sections of all 106 specimens using a BRAFV600E mutation-specific antibody (clone VE1, Ventana). Deaparaffinization, antigen retrieval, incubation in primary antibody and counterstaining was performed according to manufacturer specifications using an automated immunohistochemical stainer (Ventana Medical Systems). Appropriate positive (BRAFV600E mutant papillary thyroid carcinoma) and negative controls were included. VE1 antibody immunoexpression was independently scored by three pathologists (JW, SLA, IJS) as positive or negative. Positive cases were defined as showing diffuse strong or weak cytoplasmic staining in the majority (>50%) of neoplastic cells, with no more than focal, granular staining of occasional background stromal cells. Focal, weak nuclear staining, cytoplasmic staining of isolated tumor cells near the periphery, and confluent cytoplasmic staining of epithelial and stromal cells were considered negative. Diffuse nuclear staining of tumor cells was considered positive if encountered in the absence of stromal staining. Cases for which consensus scoring could not be achieved were considered equivocal. Immunohistochemical scoring was performed prior to molecular testing and the molecular pathologist (JMY) and laboratory technologist were blinded to IHC results.
BRAFV600E mutation analysis
BRAFV600E mutation analysis was performed on the 30 ameloblastoma specimens with adequate (>30%) neoplastic cellularity and on any KCOT and DC exhibiting positive immunohistochemical staining. DNA was extracted from unstained formalin-fixed paraffin-embedded slides according to manufacturer instructions using the Maxwell® RSC platform and the Maxwell RSC DNA FFPE Kit (Promega Corporation, Madison WI). BRAFV600E mutation analysis was performed using TaqMan™ Mutation Detection Assay with castPCR Technology on the Applied Biosystems™ 7500 Fast Real-Time PCR System (ThermoFisher Scientific, Waltham MA). Briefly, after PCR amplification, detection of target DNA was performed using oligonucleotide probes specific for BRAF-wild type and BRAFV600E mutant alleles. The presence or absence of mutation was determined based on the ratio of the fluorescent signals according to manufacturer-validated cutoffs using the Mutation Detector Software v2.0 and Applied Biosystems 7500 Software v2.3 (ThermoFisher Scientific, Waltham MA). T-test and chi-squared test were used for statistical analysis with clinicopathological parameters. A p value of ≤0.05 using a 95% confidence interval was considered statistically significant.
Results
Clinical and histopathologic characteristics
The clinical characteristics of the study cohort are summarized in Table 1. With regards to ameloblastoma, patient age ranged from 11 to 85 years (mean 51.6 y) with a male:female ratio of 1.6:1. The mandible was involved in 80.4% (37/46) of cases and the maxilla in 19.6% (9/46) of cases. Tumors ranged in size from 0.6–6.0 cm (mean 3.4 cm).
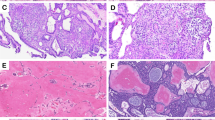
The ameloblastoma cohort comprised 36 (78.3%) conventional intraosseous ameloblastomas, 6 (13.0%) unicystic ameloblastomas, and 4 (8.7%) peripheral ameloblastomas. Dominant follicular growth was present in 55.0% (22/40) and dominant plexiform growth was present in 35.0% (14/40) of conventional/peripheral ameloblastoma; an admixture of follicular and plexiform patterns of growth was present in 10% (4/40) of those cases. Unicystic ameloblastomas were not assessed for patterns of growth as their dominant growth pattern was considered macrocystic. The most common histopathologic variants were classic (stellate reticulum-like) and acanthomatous, which frequently co-existed. Ameloblastomas containing any combination of those two variants, in the absence of any other variants, comprised 87.0% (40/46) of the cohort. Granular (6.5%, 3/46), basaloid (4.3%, 2/46) and desmoplastic (2.2%, 1/46) features were much less common and oftentimes focal. The histologic findings are summarized in Fig. 1.
BRAF VE1 IHC
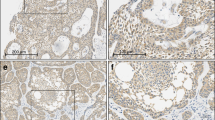
The results of BRAF VE1 IHC are summarized in Table 2. A total of 67.4% (31/46) of ameloblastomas were positive, with varying intensity from weak to strong (Figs. 2–4). One case showed peculiar diffuse nuclear positivity of tumor cells (Fig. 3a–c), interpreted as positive. A total of 4.3% (2/46) of ameloblastomas, one mandibular and one maxillary, were equivocal and 28.3% (13/46) of ameloblastomas were negative. Equivocal cases exhibited weak cytoplasmic staining in approximately half of tumor cells, and did not achieve consensus interpretation. By anatomic location, 83.8% (31/37) of mandibular ameloblastomas were positive and 0.0% (0/9) maxillary ameloblastomas were positive. No staining was identified in any KCOT (0/30, 0.0%) or DC (0/30, 0.0%) (Fig. 5).
A Unicystic ameloblastoma exhibiting macrocystic growth (case 46). B Cystic lining exhibiting subtle basal cell hyperplasia and discohesion. C Overt ameloblastic differentiation in the form of nuclear hyperchromasia of basal cells and stellate reticulum-like change of suprabasal cells is present focally. D–F Confluent BRAF VE1 immunoreactivity throughout the cystic proliferation is noted, including in areas of subtle and more overt ameloblastic differentiation. Inset: granular cytoplasmic immunoreactivity with BRAF VE1 IHC.
A–C Conventional ameloblastoma with cystic degeneration (case 6) exhibiting strong, diffuse granular positivity for BRAF VE1, restricted to tumor nuclei and in the absence of any cytoplasmic or stromal staining. D Ameloblastoma focally exhibiting desmoplastic features (case 32), in which tumor cells are compressed and hyperchromatic with minimal cytoplasm. E, F BRAF VE1 cytoplasmic granular immunoreactivity of variable intensity in areas of conventional follicular growth, but with absence of staining in areas with desmoplastic phenotype.
A, B Granular cell ameloblastoma (case 2) comprised of cells with abundant granular cytoplasm, in the absence of conventional cytologic features of ameloblastoma. C Tumor cells are positive for BRAF VE1, with weaker staining in cells with greater cytoplasmic granularity. D–F Basal cell ameloblastoma (case 17) with cortical perforation exhibiting no BRAF VE1 immunoreactivity.
A, D KCOT characterized by cystic proliferation of uniformly thin epithelium exhibiting nuclear hyperchromasia and parakeratosis; tumor cells are completely negative for BRAF VE1 IHC. B, E DC characterized by nonkeratinized, uniformly thin stratified squamous cyst lining; cyst lining is completely negative for BRAF VE1 IHC. C, F Unicystic ameloblastoma with incipient mural invasion (case 39) exhibiting very weak cytoplasmic granular staining of tumor cells, interpreted as equivocal.
The ameloblastoma cohort included 16 paucicellular cases with inadequate neoplastic cellularity for mutational analysis. These specimens were positive for BRAF VE1 IHC in 81.3% (13/16) of cases, relative to 67.4% (31/46) across the entire cohort. With regards to mandibular ameloblastomas specifically, paucicellelular specimens were positive in 92.9% (13/14) of cases, relative to 83.8% (31/37) across the entire cohort. This demonstrates good reliability of BRAF VE1 IHC even in paucicellular or inflamed specimens, in spite of a tendency for variable staining intensity.
BRAFV600E mutation analysis
BRAFV600E mutation analysis was performed on the 30 ameloblastomas with adequate neoplastic cellularity and was not performed on any KCOT or DC. The results of mutational analysis are summarized in Table 3. BRAFV600E mutation was identified in 63.3% (19/30) ameloblastomas, and was identified in all positive (18/18, 100.0%) and equivocal (1/1, 100.0%) cases by IHC. No BRAFV600E mutations were identified in any of the 11 (0.0%) cases that were negative by IHC. The sensitivity and specificity of BRAF VE1 IHC for BRAFV600E mutation was 100.0% when considering the equivocal case positive, and the sensitivity was 94.7% with the equivocal case considered negative.
BRAFV600E-mutant ameloblastoma showed a statistically significant predilection for the mandible (p = 0.00001) but showed no statistically significant associations with patterns of growth or histopathologic variants, though the association with histopathologic variant approached significance (p = 0.087) (Table 4). Of note, both ameloblastomas with basaloid features were BRAF-wild type, and the one ameloblastoma with focal desmoplastic features showed BRAF VE1 staining only in the portion with conventional follicular growth, though the number of cases with these features was too small to assess for statistical significance. The mean age at diagnosis of BRAFV600E-mutant ameloblastoma was lower than that of BRAF-wild type ameloblastoma, but this was not statistically significant (56.9 vs. 63.4 yrs, p = 0.36). The salient clinical, histopathologic, immunohistochemical and molecular features are summarized in Fig. 1.
Discussion
Over the past decade, tumor-specific genetic alterations have been increasingly identified in odontogenic neoplasms. These include MAPK pathway alterations and SMO mutations in ameloblastoma, ameloblastic fibroma, ameloblastic carcinoma and ameloblastic fibrosarcoma7,8,9,11,12,13; PTCH1 mutations in KCOT15; CTNNB1 mutations in calcifying cystic odontogenic tumor20; KRAS mutations in adenomatoid odontogenic tumor21; EWSR1 rearrangements in clear cell odontogenic carcinoma22; and FOS rearrangements in cementoblastoma23. Such findings hold promise in facilitating diagnostic accuracy and developing classification schemes in odontogenic pathology, and may support the development of immunohistochemical surrogates that prove helpful in diagnostically challenging scenarios or in the setting of lack of pathologist familiarity/experience. Ameloblastoma is uniquely aggressive among odontogenic cysts and neoplasms, requiring surgical resection with bony margins of at least 1 cm, and correct diagnosis on biopsy specimens is particularly critical for optimal patient management 24.
This study showed that the BRAF VE1 antibody is an excellent marker for mandibular ameloblastoma with 100% specificity and 83.8% sensitivity in this location in the context of important diagnostic mimics, and is highly predictive of BRAFV600E mutation. Immunohistochemical staining varied in intensity from weak to strong but in spite of this is reliable even in small biopsy specimens or specimens with limited (<30%) neoplastic cellularity. This is supported by a high rate of positivity of 92.9% in those mandibular ameloblastomas (cases 31–46), which compares favorably to the overall rate of positivity of 83.8% in mandibular ameloblastomas across the entire cohort. Weak staining in approximately half of tumor cells that was difficult to interpret as positive or negative, and scored as equivocal, was seen in only 2 (4.3%) ameloblastomas, one of which was sequenced and found to harbor BRAFV600E mutation. This suggests that reflex molecular testing for BRAFV600E mutation in diagnostically challenging cases with equivocal BRAF VE1 staining may be valuable in clinical practice. The study cohort was restricted to non-decalcified ameloblastomas following negative IHC in five previously decalcified ameloblastoma specimens, and it is important to note that BRAF VE1 IHC may be unreliable in the setting of prior decalcification.
No maxillary ameloblastomas were positive for BRAF VE1 IHC in this cohort, suggesting that this antibody is likely to be most helpful as an immunohistochemical adjunct in the workup of diagnostically challenging tumors of mandibular origin, where ameloblastoma does occur at a 5-fold greater frequency1. This limitation is to be expected, given that BRAFV600E mutation occurs in over 70% of mandibular ameloblastomas but in only 5–20% of maxillary ameloblastomas, for reasons that are not well understood25,26. The near-perfect concordance between BRAF VE1 IHC and BRAFV600E mutation in this cohort and in the ameloblastoma literature suggests that BRAF VE1 IHC may also be helpful as a screening tool for identifying ameloblastomas that could possibly benefit from BRAF-inhibition, as has been reported rarely27. More work is needed to determine whether RAS Q61R IHC, possibly in conjunction with BRAF VE1 IHC, may be beneficial in diagnostically challenging maxillary cases, since as many as 40% of maxillary ameloblastomas harbor KRAS (G12R), NRAS (Q61R and Q61K), and HRAS (Q61R, Q61K and G12S) mutations.
BRAFV600E mutant ameloblastoma in this cohort showed a statistically significant mandibular predilection, in good agreement with prior literature. BRAFV600E mutant ameloblastoma has also been shown to present 1–2 decades earlier in life than BRAF-wild type ameloblastoma, but in this study the differences in age were not statistically significant9,28. This is likely because pediatric ameloblastomas were omitted from the sequencing cohort, and it raises the hypothesis that pediatric ameloblastomas, in particular, may be enriched for BRAFV600E mutation. The impact of BRAFV600E mutation on prognosis in the literature is less clear. BRAFV600E mutation has been associated with less aggressive behavior9, more aggressive behavior29, and no impact on prognosis28, while in another study ameloblastomas with double or triple mutations were associated with highest recurrence rates26. This study cohort was not designed to assess outcomes as it consisted of biopsy specimens only. Ameloblastoma is characterized by tremendous histologic diversity including different patterns of growth (follicular, plexiform or unicystic) and different variants in terms of dominant cell type (classic/stellate reticulum-like, acanthomatous, basaloid or granular) that accompany the characteristic ameloblast-like peripheral cells1. Other rare variants also exist, including desmoplastic ameloblastoma, adenoid ameloblastoma and keratoameloblastoma. Generally speaking, these different histologic presentations have no impact on prognosis, with the important exception of unicystic ameloblastoma when it grows as a single macrocystic structure with no invasion into surrounding stroma, which may be treated more conservatively2. Adenoid ameloblastoma, additionally, appears more aggressive, and there is some discussion as to whether this represents a rare variant of ameloblastoma or a distinct entity altogether30,31. More recently, studies have attempted to determine whether histopathologic presentation may predict genotype, with variable findings. Some studies have shown that plexiform growth is significantly more common in BRAF-wild type ameloblastoma8,9,32 and that follicular growth is more common in BRAFV600E-mutant ameloblastoma26. Unicystic ameloblastoma is also highly associated with BRAFV600E mutation, in which it occurs in over 90% of cases, and in this cohort all 6 unicystic ameloblastomas were BRAFV600E-mutant10. This study failed to demonstrate, however, any statistically significant association between BRAF mutational status and histologic parameters, though findings may be limited by sample size. The tendency for ameloblastomas with classic (stellate reticulum-like) and/or acanthomatous cells to associate with BRAFV600E mutation approached statistical significance, suggesting that the most well-recognized presentation of ameloblastoma, follicular growth with classic/acanthomatous cells may be associated with BRAFV600E mutation. Additionally, both ameloblastomas with basaloid features in this series were BRAF-wild type, suggesting that, overall, specific genotype-phenotype correlations could possibly be identified in adequately powered studies. Of note, the single ameloblastoma in this cohort with desmoplastic features showed absence of BRAF VE1 staining in tumor cells compressed by desmoplastic stroma, but this is unlikely to be of any biologic significance. BRAFV600E mutation has been previously reported in desmoplastic ameloblastoma and it is likely the case that the compressed tumor cells, with high nuclear/cytoplasmic ratio, simply express insufficient cytoplasmic BRAF V600E for immunohistochemical detection 11.
One ameloblastoma in this series exhibited peculiar diffuse nuclear staining of tumor cells in the absence of stromal staining and was found subsequently to harbor a BRAFV600E mutation. Nuclear staining with the BRAF VE1 antibody has been rarely reported and is considered non-specific as it does not correlate with mutational status33,34,35. These reported cases, however, exhibited focal, weak staining of tumor nuclei, occasionally in the context of equivocal cytoplasmic/stromal staining, unlike our case. Whether diffuse nuclear staining of tumor cells, as identified in our case, occurs with any frequency and whether it consistently associates with BRAFV600E mutation or any other clinical or prognostic variable is unknown and requires additional study.
Lastly, BRAF VE1 IHC was negative in all KCOT and DC, suggesting that BRAFV600E mutation plays no role in the pathogenesis of these conditions. Regarding KCOT, this finding stands in contrast to a prior study identifying frequent BRAFV600E mutation in KCOT36 and in agreement with the findings of a large cohort of sequenced KCOTs identifying near-universal PTCH1 alterations but no BRAFV600E mutations 15.
In conclusion, BRAF VE1 IHC is an accurate diagnostic adjunct for mandibular ameloblastoma, specific for its diagnosis in the context of important mimics and concordant with BRAFV600E mutational status. Its utility in the maxilla is limited on account of low frequency BRAFV600E mutation in maxillary ameloblastomas, and even in the mandible negative staining must be interpreted with caution on account of the occurrence of occasional BRAF-wild type ameloblastoma. In challenging pathology settings BRAF VE1 IHC may contribute to correct diagnosis, and holds promise for identifying cases that may benefit from neoadjuvant therapy in the future.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Reichart, P. A., Philipsen, H. P. & Sonner, S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol 31B, 86–99 (1995).
Li, T. J., Wu, Y. T., Yu, S. F. & Yu, G. Y. Unicystic ameloblastoma: a clinicopathologic study of 33 Chinese patients. Am J Surg Pathol 24, 1385–1392 (2000).
DeVilliers, P., Liu, H., Suggs, C., Simmons, D., Daly, B., Zhang, S. et al. Calretinin expression in the differential diagnosis of human ameloblastoma and keratocystic odontogenic tumor. Am J Surg Pathol 32, 256–260 (2008).
Cairns, L., Naidu, A., Robinson, C. M., Sloan, P., Wright, J. M. & Hunter, K. D. CD56 (NCAM) expression in ameloblastomas and other odontogenic lesions. Histopathol 57, 544–548 (2010).
Kusafuka, K., Hirobe, K., Wato, M., Tanaka, A. & Nakajima, T. CD56 expression is associated with neuroectodermal differentiation in ameloblastomas: an immunohistochemical evaluation in comparison with odontogenic cystic lesions. Med Mol Morphol 44, 79–85 (2011).
Koneru, A., Hallikeri, K., Nellithady, G. S., Krishnapillai, R. & Prabhu, S. Immunohistochemical expression of calretinin in ameloblastoma, adenomatoid odontogenic tumor, and keratocystic odontogenic tumor: a comparative study. Appl Immunohistochem Mol Morphol 22, 762–767 (2014).
Kurppa, K. J., Caton, J., Morgan, P. R., Ristimaki, A., Ruhin, B., Kellokoski, J. et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol 232, 492–498 (2014).
Sweeney, R. T., McClary, A. C., Myers, B. R., Biscocho, J., Neahring, L., Kwei, K. A. et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet 46, 722–725 (2014).
Brown, N. A., Rolland, D., McHugh, J. B., Weigelin, H. C., Zhao, L., Lim, M. S. et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 20, 5517–5526 (2014).
Heikinheimo, K., Huhtala, J. M., Thiel, A., Kurppa, K. J., Heikinheimo, H., Kovac, M. et al. The mutational profile of unicystic ameloblastoma. J Dent Res 98, 54–60 (2019).
Diniz, M. G., Gomes, C. C., Guimaraes, B. V., Castro, W. H., Lacerda, J. C., Cardoso, S. V. et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol 36, 5649–5653 (2015).
Agaimy, A., Skalova, A., Franchi, A., Alshagroud, R., Gill, A. J., Stoehr, R. et al. Ameloblastic fibrosarcoma: clinicopathological and molecular analysis of seven cases highlighting frequent BRAF and occasional NRAS mutations. Histopathol 76, 814–821 (2020).
Coura, B. P., Bernardes, V. F., de Sousa, S. F., Diniz, M. G., Moreira, R. G., de Andrade, B. A. B. et al. Targeted next-generation sequencing and allele-specific quantitative PCR of laser capture microdissected samples uncover molecular differences in mixed odontogenic tumors. J Mol Diagn 22, 1393–1399 (2020).
Qu, J., Yu, F., Hong, Y., Guo, Y., Sun, L., Li, X. et al. Underestimated PTCH1 mutation rate in sporadic keratocystic odontogenic tumors. Oral Oncol 51, 40–45 (2015).
Stojanov, I. J., Schaefer, I. M., Menon, R. S., Wasman, J., Gokozan, H. N., Garcia, E. P. et al. Biallelic PTCH1 inactivation is a dominant genomic change in sporadic keratocystic odontogenic tumors. Am J Surg Pathol 44, 553–560 (2020).
Seki-Soda, M., Sano, T., Ito, K., Yokoo, S. & Oyama, T. An immunohistochemical and genetic study of BRAF(V600E) mutation in Japanese patients with ameloblastoma. Pathol Int 70, 224–230 (2020).
da Silva Marcelino, B. M. R., Parise, G. K., do Canto, A. M., Sassi, L. M., Sarmento, D. J. S., Costa, A. L. F. et al. Comparison of immunohistochemistry and DNA sequencing for BRAF V600E mutation detection in mandibular ameloblastomas. Appl Immunohistochem Mol Morphol 29, 390–393 (2021).
Ritterhouse, L. L. & Barletta, J. A. BRAF V600E mutation-specific antibody: a review. Semin Diagn Pathol 32, 400–408 (2015).
Singarayer, R., Mete, O., Perrier, L., Thabane, L., Asa, S. L., Van Uum, S. et al. A systematic review and meta-analysis of the diagnostic performance of BRAF V600E immunohistochemistry in thyroid histopathology. Endocr Pathol 30, 201–218 (2019).
Sekine, S., Sato, S., Takata, T., Fukuda, Y., Ishida, T., Kishino, M. et al. Beta-catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol 163, 1707–1712 (2003).
Coura, B. P., Bernardes, V. F., de Sousa, S. F., Franca, J. A., Pereira, N. B., Pontes, H. A. R. et al. KRAS mutations drive adenomatoid odontogenic tumor and are independent of clinicopathological features. Modern Pathol 32, 799–806 (2019).
Bilodeau, E. A., Weinreb, I., Antonescu, C. R., Zhang, L., Dacic, S., Muller, S. et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol 37, 1001–1005 (2013).
Lam, S. W., Cleven, A. H. G., Briaire-de Bruijn, I. H., Schreuder, W. H., Kroon, H. M., Savci-Heijink, D. C. et al. FOS rearrangement and expression in cementoblastoma. Am J Surg Pathol 45, 690–693 (2021).
Peacock, Z. S., Ji, Y. D. & Faquin, W. C. What is important for confirming negative margins when resecting mandibular ameloblastomas? J Oral Maxillofac Surg 75, 1185–1190 (2017).
Brown, N. A. & Betz, B. L. Ameloblastoma: a review of recent molecular pathogenetic discoveries. Biomark Cancer 7, 19–24 (2015).
Gultekin, S. E., Aziz, R., Heydt, C., Senguven, B., Zoller, J., Safi, A. F. et al. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch 472, 807–814 (2018).
Hirschhorn, A., Campino, G. A., Vered, M., Greenberg, G., Yacobi, R., Yahalom, R. et al. Upfront rational therapy in BRAF V600E mutated pediatric ameloblastoma promotes ad integrum mandibular regeneration. J Tissue Eng Regen Med 15, 1155–1161 (2021).
Bonacina, R., Indini, A., Massazza, G., Rulli, E., Gianatti, A., Mandala, M. et al. Correlation of BRAF mutational status with clinical characteristics and survival outcomes of patients with ameloblastoma: the experience of 11 Italian centres. J Clin Pathol https://doi.org/10.1136/jclinpath-2021-207527 (2021).
Fregnani, E. R., Perez, D. E., Paes de Almeida, O., Fonseca, F. P., Soares, F. A., Castro-Junior, G. et al. BRAF-V600E expression correlates with ameloblastoma aggressiveness. Histopathol 70, 473–484 (2017).
Loyola, A. M., Cardoso, S. V., de Faria, P. R., Servato, J. P., Eisenberg, A. L., Dias, F. L. et al. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol 120, 368–377 (2015).
Coura, B. P., Dos Santos, J. N., Fonseca, F. P., Bernardes, V. F., de Aquino, S. N., Jorge Junior, J. et al. Adenoid ameloblastoma with dentinoid is molecularly different from ameloblastomas and adenomatoid odontogenic tumors. J Oral Pathol Med 50, 1067–1071 (2021).
Owosho, A. A., Ladeji, A. M., Adebiyi, K. E., Olajide, M. A., Okoye, I. S. I., Kehinde, T. et al. BRAF V600E mutation-specific immunohistochemical analysis in ameloblastomas: a 44-patient cohort study from a single institution. Eur Arch Otorhinolaryngol 278, 3065–3071 (2021).
Capper, D., Berghoff, A. S., Magerle, M., Ilhan, A., Wohrer, A., Hackl, M. et al. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol 123, 223–233 (2012).
Bledsoe, J. R., Kamionek, M. & Mino-Kenudson, M. BRAF V600E immunohistochemistry is reliable in primary and metastatic colorectal carcinoma regardless of treatment status and shows high intratumoral homogeneity. Am J Surg Pathol 38, 1418–1428 (2014).
Dvorak, K., Higgins, A., Palting, J., Cohen, M. & Brunhoeber, P. Immunohistochemistry with Anti-BRAF V600E (VE1) Mouse Monoclonal Antibody is a Sensitive Method for Detection of the BRAF V600E Mutation in Colon Cancer: Evaluation of 120 Cases with and without KRAS Mutation and Literature Review. Pathol Oncol Res 25, 349-359 (2019).
Cha, Y. H., Cho, E. S., Kang, H. E., Ko, J., Nam, W., Kim, H. J. et al. Frequent oncogenic BRAF V600E mutation in odontogenic keratocyst. Oral Oncol 74, 62–67 (2017).
Acknowledgements
We thank Mr. Amad Awadallah and Mr. Cameron Norris for their excellent technical assistance. This work was supported by University Hospitals Cleveland Medical Center, Department of Pathology intradepartmental funds.
Author information
Authors and Affiliations
Contributions
L.D.M. and I.J.S. performed study concept and design and wrote the paper. S.L.A., J.W. and I.J.S. performed pathological and immunohistochemical review of the cases. L.D.M., N.S.W. and I.J.S. acquired clinicopathologic data. J.M.Y. performed acquisition and interpretation of molecular data. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The present study was approved by the Institutional Review Board of University Hospitals Cleveland Medical Center (STUDY20200628) and conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mendez, L.D., Wolsefer, N.S., Asa, S.L. et al. The diagnostic utility of BRAF VE1 mutation-specific immunohistochemistry in ameloblastoma. Mod Pathol 35, 1570–1577 (2022). https://doi.org/10.1038/s41379-022-01105-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01105-8
This article is cited by
-
Proceedings of the 2023 North American Society of Head and Neck Pathology Companion Meeting, New Orleans, LA, March 12, 2023: Odontogenic Tumors: Have We Achieved an Evidence-Based Classification
Head and Neck Pathology (2023)
-
An Unusual Gingival (Peripheral) Tumor with Features of Keratoameloblastoma with Cytologic Atypia or Possible Malignant Transformation Exhibiting ARID1A Mutation
Head and Neck Pathology (2023)