Abstract
V-domain Ig-containing suppressor of T-cell activation (VISTA) is a novel immune checkpoint protein and a potential immunotherapeutic target. However, its expression in endometrial cancer has not been clearly defined. This study aimed to investigate VISTA expression and determine its associations with clinicopathological features, molecular subtypes, programmed cell death-ligand 1 (PD-L1) expression, CD8+ T-cell count, and survival in a cohort of 839 patients with endometrial cancer. Using direct sequencing of the polymerase epsilon (POLE) exonuclease domain and immunohistochemistry for mismatch repair (MMR) proteins and p53, we stratified endometrial cancers into four molecular subtypes: POLE ultramutated, MMR-deficient, p53-mutant, and nonspecific molecular profile (NSMP). PD-L1, CD8, and VISTA were detected via immunohistochemistry. VISTA was expressed in the immune cells of 76.6% (643/839) of the samples and in the tumor cells of 6.8% (57/839). VISTA positivity in the immune cells was frequent in tumors staged I–III, those with positive PD-L1 or high CD8+ T-cell density, and those representing POLE ultramutated and MMR-deficient subtypes. Furthermore, VISTA positivity in tumor cells was more frequent in clear cell carcinoma samples. VISTA in immune cells was associated with improved survival in the entire cohort as well as in the endometrioid histology, stage I, PD-L1-negative, MMR-deficient, MMR-proficient, and high and low number of CD8+ T-cell-infiltrated tumor subgroups. VISTA in immune cells was a prognostic factor overall, as well as in patients with endometrioid histology, independent of molecular subtype or CD8+ T-cell density. The data produced by this study, which was the largest to focus on VISTA expression in patients with endometrial cancer to date, suggest that VISTA is a predictor of improved survival.
Similar content being viewed by others
Introduction
Endometrial cancer is the most prevalent gynecological cancer in developed countries and its incidence rate continues to rise1. Although great progress has been achieved in improving the outcomes of women with this malignancy, effective novel therapies beyond conventional treatment are necessary to achieve the best chances of survival. This is especially true for patients with high-grade endometrioid endometrial carcinoma (EEC) and non-EEC, who are at high risks of recurrence.
Immune checkpoint inhibitors developed over the past decade have been highly effective against numerous solid tumor types. The programmed cell death-1 (PD-1) inhibitor pembrolizumab was approved by the United States Food and Drug Administration for use in mismatch repair-deficient (MMRd) or microsatellite instability (MSI)-high solid tumors including endometrial cancer; however, given that only 20–30% of endometrial cancers exhibit MSI-high/MMRd, only a fraction of patients are eligible for this treatment. Therefore, a better understanding of the immune microenvironment is warranted to improve the response rates to PD-1 inhibitors, develop more effective agents, and improve survival in these subgroups of patients.
V-domain Ig-containing suppressor of T-cell activation (VISTA), also known as PD-1H, and VSIR, is an immune checkpoint receptor discovered in 2011, which is expressed on T cells and antigen-presenting cells, and has been shown to suppress T-cell activation, proliferation, and cytokine release2,3. In contrast to PD-1 and cytotoxic T-lymphocyte-associated antigen-4, VISTA can act as both a ligand (by binding the co-inhibitory receptor P-selectin glycoprotein ligand 1 in acidic conditions) and a receptor (by binding the ligand V-set and immunoglobulin domain containing protein 3) when regulating immune responses4,5. Furthermore, VISTA inhibition promotes anti-tumor immunity by synergizing with PD ligand 1 (PD-L1) blockade in colon cancer models6. Given its role in suppressing tumor-immune responses, two clinical trials of VISTA-blocking agents are ongoing. One is a phase I study (NCT04475523) that aims to evaluate the safety and anti-cancer activity of CI-8993, which is a human immunoglobulin G1κ monoclonal antibody targeting the VISTA ligand, in patients with relapsed/refractory solid tumors. The other is a phase I clinical trial (NCT02812875) investigating CA-170, an oral PD-L1, PD-L2, and VISTA checkpoint antagonist.
VISTA expression has been demonstrated in tumor-associated immune cells (ICs) and/or tumor cells (TCs) in different types of cancer7; e.g., it has been associated with unfavorable prognosis in patients with oral squamous cell carcinoma8. However, in contrast with its negative regulatory role in T-cell responses, increasing evidence suggests that VISTA expression on TCs or ICs correlates with improved survival among patients with ovarian, breast, colorectal, hepatocellular, and lung cancers, as well as in those with malignant pleural mesothelioma, esophageal adenocarcinoma, and craniopharyngioma9,10,11,12,13,14,15,16. Thus, the roles of VISTA in the tumor-immune microenvironment and patient prognosis require further exploration.
The Cancer Genome Atlas (TCGA) classifies endometrial cancer into four molecular subtypes with distinct prognoses: DNA polymerase epsilon (POLE) ultramutated (POLEmut), MSI hypermutated, copy number-high, and copy number-low17. In a study of 82 patients with endometrial cancer, Mulati et al.18 found that VISTA was detected in all their specimens but was not associated with patient survival. However, the association between VISTA, PD-L1, and molecular subtype remains unknown. In addition, the sample size in the study by Mulati et al.18 was small, rendering their conclusions not very persuasive. Therefore, in the present study, we aimed to investigate the expression of VISTA and its potential associations with CD8+ T cells, PD-L1, molecular subtypes, pathological features, and patient survival using a large cohort of 839 patients with endometrial cancer.
Materials and methods
Study cohort and tissue microarray construction
This retrospective study included diagnostic (curettage) and final hysterectomy specimens from patients diagnosed with grade 3 or 2–3 EEC and non-EEC between January 2009 and December 2019, or with grade 1 or 1–2 EEC between January 2014 and December 2015, at the Peking Union Medical College Hospital (Beijing, China). To determine the depth of myometrial invasion, lymphovascular space invasion (LVSI), and tumor histology and grade, hematoxylin and eosin-stained slides from diagnostic and final hysterectomy specimens were reviewed by two gynecological subspecialty pathologists (Z.H.L. and S.N.Y.), who were blinded to the original pathology reports and to each other’s interpretations. In case of disagreement, a third expert pathologist (J.C.) was consulted to arrive at a final decision. Representative areas with tumor tissue were marked on hematoxylin and eosin-stained slides and sampled for tissue microarray (TMA) blocks. TMAs with single 2 mm core per case were constructed using a tissue arrayer (MiniCore, Mitogen, Hertford, UK). Patients who received neoadjuvant chemotherapy before surgery and those with inadequate formalin-fixed and paraffin-embedded tissue blocks or TMA cores were excluded from the study. Clinical data including age at diagnosis, International Federation of Gynecology and Obstetrics (FIGO) stage, postoperative adjuvant treatment (chemotherapy and/or radiotherapy), date of recurrence or last follow-up, and patient status at the last follow-up were collected from medical records. This study was approved by the Institutional Review Board (SK-995); informed consent was not required, owing to its retrospective nature.
POLE mutation analysis
As described previously19, DNA extracted using the QiaAMP DNA micro kit (QIAGEN, Ltd, Manchester, UK) was used as a PCR template to amplify POLE exons 9–14; next, 150–200 bp products were amplified using 100 ng DNA derived from formalin-fixed, paraffin-embedded samples. Sequencing was performed using BigDye v3.1 terminator cycle sequencing chemistry on an ABI 3730 DNA analyzer (Applied Biosystems, Inc., Foster City, CA). All validated POLE mutations underwent bidirectional Sanger sequencing twice.
Immunohistochemistry
Immunohistochemistry was performed using our laboratory protocol as described previously10,11,12,20. Briefly, 4 μm TMA serial sections were deparaffinized and subjected to heat-induced epitope retrieval with 10 mM sodium citrate (pH 6.0) at 95 °C for 20 min. The endogenous peroxidase activity was quenched using a 0.3% hydrogen peroxide solution. TMA sections were incubated with primary antibodies against PD-L1 (dilution 1 : 200, clone E1L3N, Cell Signaling Technology [CST], Danvers, USA), VISTA (dilution 1 : 200, clone D1L2G, CST), CD8 (dilution 1 : 200, clone D8A8Y, CST), p53 (clone MX008, Maxim Biotechnology, Fuzhou, China), and the MMR-related proteins MSH2, MSH6, MLH1, and PMS2 (Ventana Medical Systems, Oro Valley, AZ). Human tonsil tissues treated with primary antibodies were used as positive controls for PD-L1, VISTA, and CD8 straining; stromal and inflammatory cells served as internal controls for MMR and p53, whereas the same tissues with isotype-matched antibodies comprised the negative controls. All the slides were stained using an automatic immunohistochemistry staining instrument (BOND-III; Leica Biosystems, Wetzlar, Germany) according to the manufacturer’s instructions.
Assessment of PD-L1, VISTA, and CD8
PD-L1 was evaluated using the combined positive score (CPS), which was calculated as the sum of the number of PD-L1-stained cells (TCs, lymphocytes, and macrophages) divided by the total number of viable TCs, with the quotient multiplied by 100. A CPS ≥ 1 denoted positive PD-L1 expression. After immunohistochemical staining for CD8, TMA slides were scanned using the digital slide scanner KF-PRO-020 (Kfbio, Ningbo, China). The numbers of CD8+ T cells in each TMA core were counted using Fiji, an open-source platform for biological image analysis21. The CD8+ T cells were divided into two groups according to density (high and low) using the median (18 cells per core) as the cutoff. VISTA expression was evaluated in TCs and ICs separately. TCs were considered VISTA-positive if at least 1% of cells per TMA core had membranous and/or cytoplasmic staining. The percentages of VISTA-expressing ICs were compared to those of tumor-associated ICs per TMA core and were recorded as continuous variables; ICs with ≥ 1% VISTA staining were defined as VISTA-positive, as described previously10.
Molecular subgroup assignment
DNA mismatch repair protein status was considered MMRd if the tumor exhibited a complete loss of nuclear expression of any of the MMR proteins (MLH1, PMS2, MSH2, and/or MSH6) and was considered MMR-proficient if all four MMR proteins in TCs had positive nuclear staining in the presence of an intact internal control. Mutation-type p53 staining was defined as intense and diffuse (> 70%) nuclear staining or the complete absence of protein expression in the TC nuclei; wild-type expression was defined as weak and heterogeneous staining. Endometrial cancers were categorized into the following molecular subgroups, as described previously22,23: POLEmut (tumors with pathogenic variants in the exonuclease domain of POLE), MMRd (tumors with MMRd in the absence of POLE mutation), p53-mutant (p53mut; tumors with mutation-type p53 staining in the absence of POLE mutations or MMRd), and nonspecific molecular profile (NSMP; exhibiting no POLE, MMR, or p53 alterations). These classifications are analogous to TCGA categories of POLEmut, MSI hypermutated, copy number-high, and copy number-low, respectively.
Statistical analysis
The χ2-test was used to determine the association between categorical variables. The Spearman’s rank correlation coefficient was used to determine the correlation between continuous variables. Relapse-free survival (RFS) was defined as the interval between the date of surgery and that of the detection of the first local, regional, and/or distant relapse. Disease-specific survival (DSS) was defined as the interval between the date of surgery and that of death caused by endometrial cancer. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. To identify prognostic predictors, univariate and multivariate survival analyses were performed using the Cox proportional hazards regression model and hazard ratios (HRs) with 95% confidence intervals (CIs) for recurrence and death were calculated. All statistical analyses were conducted using the Statistical Package for the Social Sciences (version 20.0; IBM Corp., Armonk, NY, USA). A two-sided P-value < 0.05 was considered statistically significant.
Results
VISTA expression in endometrial cancer
A total of 839 patients with endometrial cancer were included in this study; their median age was 58 years (range, 25–88 years). The clinicopathological characteristics of these patients are summarized in Supplementary Table S1. Molecular subgroup analysis was performed in 592 of the patients, including 451 with EEC and 141 with non-EEC. Patients received treatment according to the standard of care provided by Peking Union Medical College Hospital. Adjuvant treatment, when required, included radiotherapy (pelvic external beam radiotherapy and/or vaginal brachytherapy) with or without platinum-based chemotherapy. None of the patients received immune checkpoint inhibitors or other immunotherapeutic agents.
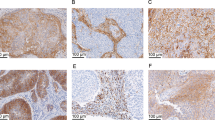
VISTA was detected in tumor-associated ICs and endometrial TCs, exhibiting a membranous/cytoplasmic staining pattern in both (Fig. 1). VISTA expression in ICs was observed in 76.6% (643/839) of the samples, whereas its expression in TCs was observed in 6.8% (57/839). Positive PD-L1 (i.e., a CPS ≥ 1) was observed in 45.1% (376/833). Of the 833 patient samples for which data regarding both proteins were available, 337 (40.5%) were double-positive for VISTA in ICs and PD-L1, 156 (18.7%) were double-negative, and 340 (40.8%) had only 1 or the other.
VISTA expression across molecular subgroups and its association with immune markers
The associations between VISTA expression in ICs/TCs and the patients’ clinicopathological characteristics, PD-L1 expression, and CD8+ T-cell density are presented in Table 1. VISTA positivity in TCs was observed significantly more frequently in samples from patients ≥58 years of age as well as that representing clear cell carcinoma. VISTA positivity in ICs was more frequent in tumors with FIGO stage I–III, positive PD-L1, high CD8+ T-cell density, and MMRd. Likewise, when evaluated as a continuous variable, the percentage of VISTA-positive ICs showed a significant positive correlation with CD8+ T-cell density (Spearman’s ρ = 0.410, P < 0.001) and the CPS of PD-L1 (Spearman’s ρ = 0.548, P < 0.001). Among the four molecular subgroups, POLEmut and MMRd tumors exhibited higher relative frequencies of positive VISTA expression in ICs, PD-L1 expression, and high CD8+ T-cell density than did NSMP and p53mut tumors (Table 1 and Supplementary Table S2). In contrast, VISTA positivity in TCs was not associated with PD-L1 expression, CD8+ T-cell density, MMR status, or molecular subtype. In addition, VISTA positivity in ICs was not associated with patient age or pathological features such as tumor histology, grade, LVSI, and myometrial invasion.
Association between VISTA expression and prognosis in patients with endometrial cancer
After excluding patients who only had curettage specimens available and those whose follow-up times were under 3 months, 666 patients who underwent total hysterectomy with complete adjuvant systemic therapy (when necessary) were subjected to survival analysis. There were no significant differences between the entire cohort of 839 patients and the subgroup that underwent survival analysis in terms of clinicopathological parameters (data not shown). After a median follow-up of 31 months (range, 4–121 months), 88 patients (13.2%) had relapsed and 64 (9.6%) had died of endometrial cancer as of July 2020.
Univariate analysis showed that age, FIGO stage, tumor grade, histology, depth of myometrial invasion, LVSI, VISTA in ICs, and molecular subgroup were significantly associated with RFS and DSS (Supplementary Table S3 and Fig. S1). VISTA in TCs, PD-L1 expression, and CD8+ T-cell density were not associated with the survival of patients overall (Supplementary Table S3). However, positive expression of VISTA in ICs was significantly associated with improved RFS (Fig. 2) and DSS (Supplementary Fig. S2) in the entire cohort. Next, patients whose molecular subtypes were known (N = 477) were subjected to multivariate analysis; there were no significant differences between this group and the remaining 189 patients with unknown molecular subtypes in terms of RFS (P = 0.686), DSS (P = 0.688), immune markers (i.e., VISTA in ICs, PD-L1, and CD8+ T cells), and clinicopathological features (age, FIGO stage, tumor grade, LVSI, and myometrial invasion), except for tumor histology (Supplementary Table S4). VISTA in ICs remained a significant survival factor in multivariate analysis, as its positivity was a predictor of improved RFS (HR = 0.55, 95% CI 0.33–0.91, P = 0.021) and DSS (HR = 0.52, 95% CI 0.29–0.93, P = 0.029) independent of molecular subtype, FIGO stage, tumor grade, or LVSI (Table 2).
Relapse-free survival (RFS) of patients A in the entire cohort, B FIGO stage I, C endometrioid endometrial carcinoma (EEC), D mismatch repair (MMR)-deficient, E MMR-proficient, F high CD8+ T-cell density, G low CD8+ T-cell density, H PD-L1-negative, and I PD-L1-positive tumor subgroups according to the expression of V-domain Ig suppressor of T-cell activation (VISTA) in immune cells.
The association between VISTA and survival was analyzed in patient subgroups stratified by FIGO stage, tumor histology, MMR status, PD-L1 status, and CD8+ T-cell density. Positive VISTA in ICs was significantly associated with improved RFS and DSS in patients with FIGO stage I (N = 447), EEC (N = 485), MMR-proficient tumors (N = 486), low CD8+ T-cell density (N = 324), high CD8+ T-cell density (N = 341), and PD-L1-negative tumors (N = 358) (Fig. 2 and Supplementary Fig. S2). VISTA in ICs was positively associated with RFS (Fig. 2), but not with DSS (Supplementary Fig. S2) in patients with MMRd tumors (N = 177). VISTA in ICs was not associated with survival in patients with FIGO stage II–IV, non-EEC, or PD-L1-positive tumors. We also evaluated the prognostic significance of VISTA expression in ICs within each molecular subtype. In patients with MMRd tumors (N = 136), VISTA expression was positively associated with RFS (P = 0.035) but not with DSS (P = 0.165); its expression was not associated with survival in patients whose tumors were of the other three molecular subtypes.
Considering that EEC is the most common subtype of endometrial cancer, we further strove to identify predictors of survival in patients with this disease. A high CD8+ T-cell density was found to be associated with longer survival in patients with EEC even though it was not associated with survival when analyzing the entire cohort (Supplementary Table S5). In univariate analysis, we found that FIGO stage, VISTA expression in ICs, CD8+ T-cell density, molecular subtype, and pathological features (tumor grade, LVSI, and myometrial invasion) were correlated with survival (Supplementary Table S5). In multivariate analysis, VISTA positivity in ICs was a predictor of improved RFS and DSS, independent of FIGO stage, pathological features, or CD8+ T-cell density (Table 3).
Discussion
To our knowledge, ours was the largest study to date on the role of VISTA in patients with endometrial cancer and the first to examine its expression in individual molecular subtypes of this disease. VISTA expression was detected in both TCs and ICs, predominantly in the latter. VISTA positivity in ICs was observed significantly more frequently in early-stage tumors, as well as in those exhibiting PD-L1 positivity, high CD8+ T-cell density, and MMRd and POLEmut subtypes; its IC positivity was found to be associated with a favorable prognosis and was a predictor of improved survival independent of FIGO stage, molecular subtype, CD8+ T-cell density, or MMR status.
VISTA is not expressed in most solid tissues, except the tonsil, spleen, and placenta. However, it is present in many cancers such as ovarian, pancreatic, gastric, lung, breast, prostate, colorectal, cervical, and endometrial cancers, as well as hepatocellular carcinoma, craniopharyngioma, gestational trophoblastic neoplasia, and melanoma8,9,10,11,12,13,14,15,16,18,20,24,25,26,27,28. Mulati et al.18 observed tumoral VISTA expression in all of their 82 endometrial cancer specimens. However, they found VISTA expression in the T cells of only four samples18. In the present study, VISTA expression was detected in both TCs (6.8%) and ICs (76.6%), predominantly in the latter, which was consistent with its expression patterns in breast, colorectal, and gastric cancers, as well as esophageal adenocarcinoma10,12,15,25. In addition, VISTA in TCs was observed more frequently in clear cell carcinoma (29.8%) than in other histological types. The discrepancy between our and Mulati et al.’s18 findings may be explained by different anti-VISTA antibodies used for immunohistochemistry, varying staining techniques, and the definition of VISTA positivity. To date, only two studies including ours have investigated VISTA in endometrial cancer; as such, additional studies are warranted to improve our understanding of the role of the immune microenvironment in this disease.
The association between VISTA, MMR status, CD8, and PD-L1 has been investigated previously in other cancer types. VISTA expression in ICs was associated with PD-L1 in ovarian, gastric, and lung cancers, as well as in craniopharyngioma9,11,16,25. In our previous study of breast cancer, VISTA in ICs correlated with PD-1, PD-L1, CD8, and tumor-infiltrating lymphocytes12, and in our study of colorectal cancer, high VISTA in ICs was observed more frequently in tumors with MMRd and high CD8+ T-cell density10. Consistent with findings in other cancers, VISTA in ICs was positively correlated with PD-L1, high CD8+ T-cell density, and MMRd in endometrial tumors. As with PD-L1 and CD8+ T cells, VISTA in ICs was more frequent in POLEmut and MMRd molecular subtypes, which were characterized by ultramutated and/or hypermutated tumors, high neoantigen loads, and robust tumor-infiltrating lymphocyte responses17,29,30. In addition, high VISTA expression in ICs was observed more often in KRAS- and PIK3CA-mutant gastric cancers and in BRAF-mutant craniopharyngioma9,25. Although the tumor mutation burden was not directly measured, the associations between VISTA and certain gene alterations that are linked to mutational burdens indicate that the tumor mutation load and/or molecular features influence either the expression of VISTA in ICs or the infiltration of VISTA-positive ICs into tumors. However, the regulatory mechanism of VISTA and its association with tumor mutational burden remain unknown; as such, future studies are warranted to identify and validate VISTA expression patterns and regulatory mechanisms in endometrial cancer, as this may assist in guiding immunotherapeutic interventions.
VISTA has been identified as a co-inhibitory molecule that suppresses T-cell-mediated immunity and promotes immune escape2,3. VISTA expression was found to be a poor prognostic factor in patients with oral squamous cell carcinoma8 and in those with primary cutaneous melanoma24. However, increasing evidence suggests that VISTA correlates with improved survival in patients with numerous other types of cancer31. Consistent with our findings in breast and colorectal cancer10,12, VISTA expression in ICs, but not in TCs, was associated with early-stage disease and improved survival in patients with endometrial cancer, and was a prognostic factor independent of tumor stage or molecular subtypes. Molecular subtypes as categorized by TCGA have proved to be strong prognostic indicators and molecularly integrated prognostic risk groups have been used to guide adjuvant treatment for patients with endometrial cancer23,32. In a study of 460 endometrial cancer specimens, Talhouk et al.30 found that the “proactive molecular risk classifier in endometrial cancer” molecular subtype was the only independent prognostic factor in their multivariable models that also incorporated T-cell markers and tumor-infiltrating lymphocyte clusters as analytical variables. In contrast to Talhouk et al.’s30 results, we found both molecular subtype and VISTA expression in ICs had prognostic value on multivariable analysis. Furthermore, VISTA in ICs was associated with survival in patients with MMR-proficient and MMRd tumors. Our data suggest that VISTA in ICs is a predictor of improved RFS independent of molecular subtype. Therefore, the VISTA-based immunoscore, just like the T-cell immunoscore in colon cancer33, may be taken into consideration for endometrial cancer risk classification once the prognostic value of VISTA is validated widely. Importantly, the association between VISTA expression and favorable prognosis suggests that using anti-VISTA therapy to treat cancer may be detrimental to the patients. Further research is required to clarify the roles of VISTA in different cancers, including endometrial cancer.
Although VISTA expression in ICs was positively correlated with CD8+ T-cell density, VISTA remained associated with RFS in patients with both high and low numbers of CD8+ T-cell-infiltrated tumors. In subgroup analysis of EEC, both VISTA in ICs and CD8+ T-cell density were found to be predictors of survival, suggesting that the prognostic value of VISTA is independent of CD8+ T cells. Previous studies, including ours in colorectal cancer, showed that VISTA was predominantly expressed on myeloid-derived cells such as CD11b+, CD45+, and CD14+ cells, as well as CD68+ macrophages in the peripheral blood and tumor microenvironment10,34,35,36,37. This may explain the prognostic value of VISTA in patients exhibiting a low number of CD8+ T-cell-infiltrated tumors.
The strengths of our study included that it was the first to investigate VISTA across different endometrial cancer molecular subtypes and its prognostic value in such a large cohort of patients with this disease who were treated at a tertiary hospital. However, there were also some limitations. First, it was a retrospective investigation that had inherent, unavoidable biases. Second, the TMAs may not have accurately represented the entirety of each sample given intratumoral heterogeneity. Third, our study was limited by its single-center nature and the lack of an independent validation cohort. Furthermore, the co-localization of VISTA and IC subgroups was not analyzed; hence, future studies that use multiplex immunofluorescence methods are warranted to elucidate the expression and function of VISTA within the tumor-immune microenvironment.
In conclusion, our assessment of the expression of VISTA in a large cohort of patients with endometrial cancer revealed that this protein is expressed in ICs and TCs, predominantly in the former. We found that VISTA positivity in ICs, but not in TCs, was more frequent in tumors representing early-stage disease, PD-L1 positivity, high CD8+ T-cell density, and POLEmut and MMRd subtypes. Moreover, VISTA in ICs was associated with a favorable prognosis independent of molecular subtype or CD8+ T-cell density. Given that our data suggest that VISTA expression is a predictor of improved survival, its inhibition may be counterproductive for patients with endometrial cancer. This ought to be considered in any future trials of VISTA-modulating immunotherapy for patients with this disease. Further research is required to clarify the underlying regulatory mechanisms of VISTA and its biological roles in endometrial cancer.
Data availability
The raw data used in this study are available from the corresponding author upon reasonable request.
References
Morice, P., Leary, A., Creutzberg, C., Abu-Rustum, N. & Darai, E. Endometrial cancer. Lancet 387, 1094–1108 (2016).
Wang, L. et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 208, 577–592 (2011).
Flies, D. B. et al. Coinhibitory receptor PD-1H preferentially suppresses CD4(+) T cell-mediated immunity. J. Clin. Invest. 124, 1966–1975 (2014).
Wang, J. et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 156, 74–85 (2019).
Johnston, R. J. et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 574, 565–570 (2019).
Liu, J. et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl Acad. Sci. USA 112, 6682–6687 (2015).
ElTanbouly, M. A., Schaafsma, E., Noelle, R. J. & Lines, J. L. VISTA: coming of age as a multi-lineage immune checkpoint. Clin. Exp. Immunol. 200, 120–130 (2020).
Wu, L. et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol. Immunother. 66, 627–636 (2017).
Wang, Y. et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and VISTA in craniopharyngioma. J. Immunother. Cancer 8, e000406 (2020)
Zong, L. et al. High VISTA expression correlates with a favorable prognosis in patients with colorectal cancer. J. Immunother. 44, 22–28 (2021).
Zong, L., Zhou, Y., Zhang, M., Chen, J. & Xiang, Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol. Immunother. 69, 33–42 (2020).
Zong, L. et al. Expression of the immune checkpoint VISTA in breast cancer. Cancer Immunol. Immunother. 69, 1437–1446 (2020).
Zhang, M. et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer 18, 511 (2018).
Muller, S. et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod. Pathol. 33, 303–311 (2020).
Loeser, H. et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology 8, e1581546 (2019).
Villarroel-Espindola, F. et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin. Cancer Res. 24, 1562–1573 (2018).
Cancer Genome Atlas Research Network et al. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013).
Mulati, K. et al. VISTA expressed in tumour cells regulates T cell function. Br. J. Cancer 120, 115–127 (2019).
Yu, S. et al. Detection of POLE subtypes in high-grade endometrioid carcinoma by BaseScope-ISH assay. Front. Oncol. 9, 831 (2019).
Zong, L. et al. B7-H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology 75, 421–430 (2019).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Bosse, T. et al. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am. J. Surg. Pathol. 42, 561–568 (2018).
Vermij, L., Smit, V., Nout, R. & Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 76, 52–63 (2020).
Kuklinski, L. F. et al. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol. Immunother. 67, 1113–1121 (2018).
Boger, C., Behrens, H. M., Kruger, S. & Rocken, C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: a future perspective for a combined gastric cancer therapy? Oncoimmunology 6, e1293215 (2017).
Gao, J. et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 23, 551–555 (2017).
Blando, J. et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl Acad. Sci. USA 116, 1692–1697 (2019).
Zong, L. et al. Expression and significance of immune checkpoints in clear cell carcinoma of the uterine cervix. J. Immunol. Res. 2020, 1283632 (2020).
Howitt, B. E. et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 1, 1319–1323 (2015).
Talhouk, A. et al. Molecular subtype not immune response drives outcomes in endometrial carcinoma. Clin. Cancer Res. 25, 2537–2548 (2019).
Yum, J. I. & Hong, Y. K. Terminating cancer by blocking VISTA as a novel immunotherapy: hasta la vista, baby. Front. Oncol. 11, 658488 (2021).
Concin, N. et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 31, 12–39 (2021).
Pagès, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018).
Xie, S. et al. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol. Immunother. 67, 1685–1694 (2018).
Le Mercier, I. et al. VISTA regulates the development of protective antitumor immunity. Cancer Res. 74, 1933–1944 (2014).
Lines, J. L. et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 74, 1924–1932 (2014).
Edwards, J. et al. Prevalence and cellular distribution of novel immune checkpoint targets across longitudinal specimens in treatment-naive melanoma patients: implications for clinical trials. Clin. Cancer Res. 25, 3247–3258 (2019).
Acknowledgements
We thank the medical record room staff for their assistance in retrieving the patients’ medical records. This work was supported by grants from the National Natural Science Foundation of China (numbers 81772783, 81971475, and 82001664), National Scientific Data Sharing Platform for Population and Health (NCMI–YF01N–201906), Chinese Academy of Medical Sciences Initiative for Innovative Medicine (numbers CAMS-2017-I2M-1-002 and CAMS-2016-I2M-1-001), and Beijing Kanghua Foundation for the Development of Traditional Chinese and Western Medicine (KH-2021-LLZX-007). The funders of the study had no role in the design of the study; collection, analysis, and interpretation of the data, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
S.Y., Y.X., and J.C. made substantial contributions to the conception and design, acquisition of data, and critical revision of the manuscript. Z.S. and S.M. made substantial contributions to patient selection and clinical data. L.Z., S.Y., and Z.L. made substantial contributions to reviewing pathological parameters, assessing immunohistochemistry results, interpreting the data, and drafting the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study was approved by the Institutional Review Board of Peking Union Medical College Hospital (approval number: S-K995).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zong, L., Mo, S., Sun, Z. et al. Analysis of the immune checkpoint V-domain Ig-containing suppressor of T-cell activation (VISTA) in endometrial cancer. Mod Pathol 35, 266–273 (2022). https://doi.org/10.1038/s41379-021-00901-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00901-y
This article is cited by
-
Expression patterns of novel immunotherapy targets in intermediate- and high-grade lung neuroendocrine neoplasms
Cancer Immunology, Immunotherapy (2024)
-
Identification and validation of TNFRSF4 as a high-profile biomarker for prognosis and immunomodulation in endometrial carcinoma
BMC Cancer (2022)
-
IGSF11 and VISTA: a pair of promising immune checkpoints in tumor immunotherapy
Biomarker Research (2022)
-
Expression of B7 family checkpoint proteins in cervical cancer
Modern Pathology (2022)