Abstract
Lobular carcinoma in situ (LCIS) is currently classified as classic (CLCIS), florid (FLCIS), and pleomorphic (PLCIS). Given the rarity of FLCIS and PLCIS, information on their clinico-pathologic features and biologic potential remains limited. We evaluated the upgrade rates at excision of FLCIS and PLCIS diagnosed on inhouse core needle biopsy (CNB) and their clinical presentation and follow-up. Over a period of 11 and a half years, there were a total of 36 inhouse CNBs with pure PLCIS (n = 8), FLCIS (n = 24), or LCIS with pleomorphic features (LCIS-PF) (n = 4). The upgrade rates to invasive carcinoma or ductal carcinoma in situ (DCIS) were 25% for PLCIS (2/8), 17% for FLCIS (4/24), and 0% for LCIS-PF (0/4). The overall upgrade rate of PLCIS and FLCIS combined was 19% (6/32). All but one case (not upgraded at excision) were radiologic–pathologic concordant. Apocrine features, previously reported only in PLCIS, were also noted in FLCIS. HER2 overexpression was seen in 13% of cases. This study highlights the more aggressive biologic features of PLCIS and FLCIS compared to CLCIS and supports surgical management for these lesions.
Similar content being viewed by others
Introduction
The morphologic spectrum of lobular carcinoma in situ (LCIS) includes classic type (CLCIS), florid (FLCIS), and pleomorphic (PLCIS) [1]. These lesions share dysregulation in the cadherin–catenin cell adhesion complex, which translates into loss of or aberrant expression of E-cadherin on the cell membrane that accounts for the characteristic non-cohesive pattern of LCIS [2]. CLCIS, first described by Foote and Stewart in 1941 as the morphologic precursor of invasive lobular carcinoma [3], is currently considered both a risk indicator and nonobligate precursor for breast cancer [4,5,6]. Contemporary studies have shown that when CLCIS is the highest risk finding on core needle biospy (CNB) excision can be spared if the pathologic and imaging findings are concordant [7,8,9,10]. PLCIS [1, 11] and more recently FLCIS [1] have been recognized as morphologic subtypes of LCIS but their clinical behavior and optimal management have not been fully characterized.
PLCIS was first described by Frost et al. in 1996 [12], as an in situ component morphologically similar to pleomorphic invasive lobular carcinoma (P-ILC) in the breast excision specimen from a 78 year-old patient who presented with mammographic calcifications. In 2000, Middleton et al. [13] reported concurrent PLCIS in 45% of cases with P-ILC. In 2012 PLCIS was introduced as a distinct variant of LCIS in the WHO classification of tumors of the breast [11].
In 2006, Fadare et al. first described a form of LCIS with central necrosis, showing morphologic features of what is now called FLCIS with necrosis [1, 14]. In their series of 18 cases, 67% were associated with invasive carcinoma [14]. The 5th edition of the World Health Organization Classification of Tumors of the Breast has defined histologic criteria for FLCIS and PLCIS [1].
In prior series the upgrade rate at excision of variant LCIS diagnosed on core needle biopsy ranged from 12–64% [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. However, many of these series did not include information on pathologic–radiologic correlation and/or independent pathologic review. In addition, most of these series focused on PLCIS and it is unclear if FLCIS was examined. We sought to assess the clinicopathologic features and upgrade rate at excision of PLCIS and FLCIS diagnosed on CNB at our institution.
Materials and methods
Study cohort
We searched our pathology database for inhouse CNBs obtained between January 2007 to June 2019 with a diagnosis of “carcinoma in situ with ductal and lobular features”, or “lobular carcinoma in situ” and the following keywords: “pleomorphic”, “massive acinar expansion”, and/or “necrosis”. These keywords were commonly used in the pathology reports at our institution to characterize the morphologic features of the LCIS subtypes now classified as FLCIS and PLCIS. Patients with concurrent ipsilateral (micro)invasive carcinoma, DCIS, atypical ductal hyperplasia (ADH), no follow-up excision, or no available slides were excluded. Cases were reviewed by at least two breast pathologists (MGK, MPM, and/ or EB) and reclassified according to the WHO 5th edition criteria as CLCIS, PLCIS, and FLCIS, with an additional descriptive category of LCIS with pleomorphic features (LCIS-PF) (Table 1). All lesions consisted of dyscohesive cells filling acini and/or ducts. CLCIS showed involvement and expansion of at least 50% of the terminal duct lobular unit by a proliferation of type A and/or type B cells. Type A cells were small with uniform hyperchromatic nuclei, whereas type B cells showed slightly larger nuclei with mild variation in size and shape, and small nucleoli. Single cell necrosis/apoptosis or minute foci of necrosis could be seen, but no comedonecrosis was present. FLCIS also consisted of type A and/or type B cells with >40 cells across the diameter of the expanded acini and ducts, and/or little to no intervening stroma between the distended acini. PLCIS showed nuclear atypia similar to high grade DCIS with nuclear size at least four times that of mature lymphocytes. LCIS with pleomorphic features showed predominantly CLCIS type B cells with scattered cells having pleomorphic nuclei.
The following features were also evaluated in each case: apocrine morphology (abundant eosinophilic, granular cytoplasm with round, often central nucleus and prominent nucleoli), necrosis (defined as more than single cell necrosis/apoptosis or minute foci), and calcifications. If PLCIS and FLCIS coexisted in the same CNB, the case was classified as PLCIS. A breast radiologist (KC) reviewed all pertinent imaging studies. Histologic and imaging correlation was assessed for all cases. Upgrade was defined as (micro)invasive carcinoma or DCIS at excision. The extent of PLCIS/FLCIS on excision was estimated based on number of tissue slices involved on excision specimens, where the exact tissue thickness can be calculated, or measuring the lesion on single glass slide or full face sections for mastectomies. If the extent of PLCIS/FLCIS was larger on CNB, this measurement was used. Clinical data were obtained from the electronic medical records. The Institutional Review Board approved the study.
Immunohistochemistry for ER, HER2, AR, and E-cadherin
Immunohistochemistry was performed in whole tissue sections for cases with available material using the following antibodies and dilutions: estrogen receptor (ER)(clone 6F11, prediluted, Leica Byosystens, Newcastle Upon Tyne, UK); androgen receptor (AR)(clone M4070, 1:250, Spring Bioscience, Pleasanton, CA), human epidermal growth factor receptor 2 (HER2)(clone 4B5, 1:2, Ventana Medical Systems, Tucson, AZ), E-cadherin (clone 36, 1:2, Ventana Medical Systems, Tucson, AZ), and a a triple stain antibody cocktail (CK HMW clone 34βE12, p63 clone 4A4, CK7 clone BC1, CK8/18 clone 5D3, prediluted, Biocare Medical, Pacheco, CA). Antigen retrieval was performed with BOND epitope retrieval solution 1 for ER, BOND epitope retrieval solution 2 for AR and triple stain, and CC1 for HER2 and E-cadherin. The triple stain was performed on selected cases to rule out microinvasion. ER and HER2 were scored on invasive carcinoma and LCIS using ASCO/CAP guidelines [30, 31]. ER and HER2 were performed on LCIS for this study only and not for clinical purposes. ER staining in at least 1% of the tumor cells was classified as a positive result. ER expression in 1–10% nuclear expression of the tumor nuclei was categorized as low positive. AR was classified as positive if nuclear staining was present in at least 1% of lesional cells. E-cadherin expression was classified as negative if no staining was present, or aberrant if the cell membrane showed granular/incomplete or reduced staining compared to adjacent normal ductal epithelium [2, 32, 33]
Dual-in-situ-hybridization (DISH) for HER2
Cases with HER2 IHC equivocal results were subjected to the INFORM HER2 Dual ISH DNA Probe Cocktail assay as per the manufacturer’s validated and recommended protocol (Catalog #780-4422, Ventana Medical Systams, Tucson, AZ). At least 20 lesional cells were manually counted, and amplification status was assigned as per ASCO/CAP 2018 guidelines [31].
Statistical analysis
Statistical analysis was performed using Fisher’s exact test and two-tailed t-test. A P value <0.05 was considered significant.
Results
Patient cohort
We identified 218 inhouse CNBs with CIS or LCIS and “pleomorphic”, “massive acinar expansion”, and/or “necrosis” out of ≈29,800 CNBs performed in our search period. Of these cases, 163 had a concurrent ipsilateral diagnosis of ADH, DCIS, or (micro)invasive carcinoma, 11 had no subsequent excision or were lost to follow-up, and eight CNBs had no available material for review. Our final study cohort consisted of 36 matched CNB-excision specimens from 35 women, including one patient with two ipsilateral breast lesions at different sites that were excised separately (see Fig. 1). The median age at diagnosis was 61 years (range 45–79). Fifteen patients (15/35, 43%) had a personal history of breast cancer (six ipsilateral and nine contralateral), four of CLCIS (4/35, 11%) and one of ipsilateral PLCIS (1/35, 3%).
Imaging and histologic findings on Core Needle Biopsy
The most common method of biopsy was stereotactic (n = 25), followed by MRI-guided (n = 8) and ultrasound-guided (n = 3). The majority of CNB were performed for suspicious mammographic calcifications (24/36, 67%); other biopsy targets included non-mass enhancement (8/36, 22%), masses (3/36, 8%), or architectural distortion (1/36, 3%). Biopsies were performed with 9–12 gauge needles. The number of tissue cores obtained ranged from 3 to 14 (mean number of cores = 8).
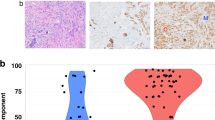
Upon re-review of the CNB cases, there were eight (22%) PLCIS, 24 (67%) FLCIS and four (11%) LCIS-PF. Apocrine features were seen in 5/8 (63%) PLCIS and 6/24 (25%) FLCIS (Fig. 2). Necrosis was present in 63% PLCIS and 71% FLCIS. Negative or aberrant E-cadherin expression was confirmed in all cases except one, in which LCIS-PF spanning 1 mm was present in the initial CNB slides, but the focus was not present in deeper sections used for immunohistochemical stains; follow-up excision of this lesion yielded ALH. One case was considered radiologic–pathologic discordant. Imaging showed an irregular mass on screening mammogram; the CNB had FLCIS with apocrine features and necrosis; no upgrade was identified at excision. All remaning 35 cases were deemed concordant.
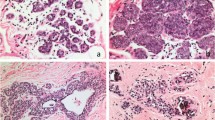
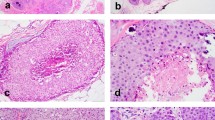
A,B CLCIS showing a dyscohesive proliferation of type A and B cells expanding more than 50% of the acini. C FLCIS with massive acinar expansion and central necrosis. D FLCIS with apocrine features, showing marked expansion of the acini by dyscohesive cells with ample granular eosinophilic cytoplasm and minimal nuclear atypia. E PLCIS is composed of a dyscohesive proliferation of cell with marked nuclear pleomorphism. F PLCIS with apocrine features. G LCIS-PF consists predominantly of type B cells with scattered cells showing enlarged and pleomorphic nuclei.
Upgrade rate at excision
The combined upgrade rate of PLCIS and FLCIS was 19% (6/32). Table 2 summarizes the clinicopathologic features and upgrade rates. The CNBs with upgrade at excision showed 2 PLCIS with apocrine features and necrosis, and four cases of FLCIS with necrosis. The clinicopathologic findings of upgraded cases are detailed in Table 3. The radiologic target was calcifications in all cases, and all calcifications were associated with the FLCIS or PLCIS at CNB. The cases with upgrade had a higher number of tissue cores removed at CNB (mean number of cores 10 vs. 7; p = 0.034). Menopausal status, breast cancer history, use of different imaging modalities between detection/diagnosis and biopsy, mean size of lesion on imaging, radiologic calcifications, and apocrine features did not correlate with upgrade, but necrosis showed a positive trend (p = 0.062, Table 4). We found that while the size of the lesion was slightly overestimated on imaging, the mesaurement was comparable to the estimated size of PLCIS/FLCIS at excision (mean size 1.48 vs. 1.31 cm; p = 0.507). The size of the radiologic target did not correlate with upgrade, however, we found that upgraded cases at excision were more likely to be associated with extensive PLCIS or FLCIS (mean size 3.2 cm vs. 0.9 cm; p = 0.001).
For the purposes of this study, deeper H&E levels were performed in all cases. A triple stain (brown chromogen = p63 and basal cytokeratins CK5 and CK14; red chromogen = luminal cytokeratins CK7 and CK18) was performed in a subset of cases with suspicious findings on H&E to rule out the presence of microinvasive carcinoma. In three cases, microinvasive lobular carcinoma was not present in initial CNB slides, but was identified in the deeper sections obtained for immunohistochemical studies. Two of these three CNBs contained FCLIS with necrosis and were upgraded to ILC (0.4 cm) and LCIS with microinvasion at excision, respectively. In the third case, the CNB had FLCIS, and no DCIS or invasive carcinoma was identified at excision. The patient had a prior history of contralateral BC and was treated with anastrozole at the time of the index CNB and subsequently. At a follow-up time of 38 months she had no evidence of recurrence or disease progression.
None of the four cases of LCIS-PF at CNB were upgraded at excision.
Treatment and follow-up
Five of six patients with upgrade at excision underwent lumpectomies; three had axillary staging with sentinel lymph node biopsy, all of which were negative. Two of three patients with ER positive (micro)invasive carcinoma received endocrine therapy (one patient refused treatment). Three patients underwent radiation therapy. One patient did not receive any adjuvant treatment post-lumpectomy (age 75 at diagnosis with microinvasive carcinoma and PLCIS on excision; receptor status unknown). The sixth patient underwent mastectomy with negative sentinel lymph nodes and received endocrine therapy for ER positive microinvasive carcinoma.
Thirty patients did not have an upgrade at excision. One of these patients underwent radiation therapy after lumpectomy for a diagnosis of “carcinoma in situ with mixed ductal and lobular features with reduced E-cadherin membranous expression”; CLCIS was also present. Upon re-review the carcinoma in situ was reclassified as FLCIS with aberrant E-cadherin expression. Fourteen of the 30 patients with no upgrade at excision were treated with endocrine therapy. Only five of the 14 patients received endocrine therapy for LCIS, while the remaining patients had a prior or concurrent contralateral diagnosis of invasive carcinoma or DCIS. The median follow-up time was 37.5 months. Three patients subsequently presented with ipsilateral (micro)invasive carcinoma (Table 5) Patient 1 was a 73 year-old woman with no personal or family history of breast cancer. She had FLCIS (ER positive, HER2 status not available) on CNB. Excision yielded PLCIS (spanning 1.0 cm) with >0.5 cm margin clearance for which she received no additional treatment. At 24 months an ipsilateral area of linear non-mass enhancement was noted on MRI. CNB showed microinvasion and FLCIS (receptors not available for microinvasive carcinoma). No residual microinvasive carcinoma was present in the lumpectomy specimen (only CLCIS present) and the sentinel lymph nodes were negative. She did not receive radiation treatment. The patient remained disease-free 87 months after the index CNB. The second patient was a 75 year-old female with a history of contralateral DCIS 9 years prior treated with excision alone (no radiation or hormonal therapy) and ipsilateral PLCIS 2 years prior status post excision. The index CNB showed PLCIS (ER positive, HER2 negative); excision yielded PLCIS with microinvasion (receptors not available for microinvasive carcinoma). PLCIS was <0.1 cm from margin. The patient did not receive radiation or hormone therapy. Fifteen months later she presented with calcifications in the same breast, CNB showed PLCIS and subsequent excision PLCIS with microinvasion, focally involving margins. She recurred with a 2.6 cm P-ILC (ER negative, PR negative, HER2 negative), DCIS, PLCIS, and FLCIS 29 months from initial diagnosis. She completed chemotherapy after excision. She subsequently died of disease 73 months after the index biopsy. The third patient was a 57 year-old who underwent excision, radiation therapy and hormone therapy (tamoxifen, later changed to anastrozole and then raloxifene) for an ipsilateral tubular carcinoma 8 years prior to the index CNB. She was diagnosed with FLCIS (ER positive, HER2 negative) on CNB; underwent excision showing FLCIS present 0.1 mm from the closest margin. She received no radiation therapy but continued raloxifene. The patient recurred after 22 months with a 0.15 cm P-ILC (ER positive, PR negative, HER2 positive), PLCIS, and FLCIS at the site of the prior FLCIS. The patient remained disease free 65 months after the index CNB.
Receptor profile on CNB
The majority of FLCIS and PLCIS cases in our study were ER positive (26/32, 81%). Apocrine features were more common in ER negative than ER positive cases (45% vs. 5%, respectively; p = 0.01). Androgen receptor was detected in all 32 cases. HER2 was positive by IHC (3+) in 4/31 cases (13%), including three with apocrine features; 4/31 (13%) cases were HER2 IHC equivocal (2+), and 23 (74%) HER2 negative (0 or 1+). All HER2 equivocal cases (three FLCIS and one PLCIS) showed apocrine features and were negative by HER2 Dual ISH (Table 6).
Discussion
Classic LCIS is both a high risk lesion and a nonobligate precursor of invasive carcinoma. Modern management is non-surgical with the use of endocrine chemoprevention to reduce the risk of subsequent invasive carcinoma or DCIS. In recent years, the morphologic spectrum of LCIS has expanded to include PLCIS and FLCIS, two uncommon morphologic subtypes. Contemporary data on FLCIS and PLCIS is limited, but suggests that these morphologic subtypes of LCIS are more frequently associated with invasive carcinoma or DCIS than CLCIS [21, 29, 34,35,36] and have genomic alterations that are usually associated with more aggressive behavior in the context of breast cancer, such as ERBB2 and ERB3 alterations [35, 36], defying classification as “benign” entities. However, the AJCC staging manual, 8th edition and the National Comprehensive Cancer Network guidelines [37, 38] regard LCIS as a benign entity with no distinction between CLCIS and the morphologic subtypes FLCIS and PLCIS. The latest ESMO guidelines for early breast carcinoma acknowledge that “The pleomorphic variant of lobular neoplasia may behave similarly to DCIS and should be treated accordingly, after multidisciplinary discussion.” but do no mention FLCIS [39]. There is lack of consensus on the management of these unusual subtypes of LCIS, including the need for surgical management, and adjuvant radiation or hormonal treatment [18, 22, 39, 40].
In this study, we reclassified LCIS with nonclassic morphology diagnosed on CNB from 2007 to 2019 using the WHO 2019 consensus criteria [1], which introduced specific criteria for the diagnosis of FLCIS. Overall, CNBs with FLCIS and PLCIS without associated ADH, DCIS, or invasive carcinoma were very rare, and accounted for approximately only one in a thousand of consecutive inhouse CNBs in the same study period. Compared to the rate of inhouse CNBs with only CLCIS, which in isolation also constitutes a rare finding, CNBs with FLCIS or PLCIS were at least 10 times less frequent.
In a prior retrospective study at our center, the upgrade rate of radiologic–pathologic concordant CNBs with classic LCIS was 3% [7]. In contrast, the combined rate of upgrade at excision of FLCIS and PLCIS was 19%, 25% for PLCIS, and 17% for FLCIS, and all cases with upgrade had concordant imaging and pathologic findings. Prior series have reported upgrade rates ranging from 12 to 64% [15,16,17,18,19,20, 22,23,24,25,26,27,28,29]. Many of these studies did not include information on radiologic–pathologic concordance, or an independent review of the slides to confirm diagnosis/classification, which may account for the wide range of upgrade rates. In addition, most series published before 2019 classified all cases as PLCIS, even though they included lesions that today qualify as FLCIS [41]. Only few groups have used diagnostic criteria consistent with the criteria recently codified in the WHO 2019 Classification of Tumors of the Breast [1] and reported separately the upgrade rates of FLCIS and PLCIS [10, 21, 29].
In the few studies that correlated imaging and CNB findings, cancer was found in 23 of 55 cases (42%) with four discordant cases among upgraded cases (4/23, 17%) [9, 16,17,18, 29]. The most common imaging finding at presentation were calcifications, with very few lesions presenting as masses or abnormal MRI enhancement [9, 15, 17, 18, 22, 28], similar to our cohort. It is worth noting that in the study by Carder et al. three of 10 upgraded cases of pure PLCIS on biopsy presented as radiologic calcifications and were deemed radiologic–pathologic concordant [17]. Two of the upgraded cases showed invasive lobular carcinoma at excision, ranging from 0.9 to 1.2 cm, neither of which had been detected on diagnostic imaging. These observations emphasize the importance of recognizing the morphologic subtypes of LCIS on CNB and support excision of these lesions for complete histologic evaluation.
The WHO 2019 recommends classifying LCIS composed predominantly of type B cells and few scattered large cells having large pleomporphic nuclei as classic LCIS with type B cells. No specific evidence is provided to support this recommendation, but this is a practical and reasonable approach to the classification of LCIS with ambiguous features in surgical excision specimens. Whether this recommendation should also be extended to the diagnosis of CNB material is unclear as no literature is available on this issue. We identified four CNBs with LCIS composed of type B cells with scattered pleomorphic nuclei. All four cases were radiologic–pathologic concordant. At excision none of these cases yielded an upgrade, and none of the patients developed carcinoma at follow-up. Although our results are compatible with the WHO 2019 recommendations, the number of CNB cases is limited and we cannot make a definitive statement on whether surgical excision can be spared in these cases.
In our series, the mean number of tissue cores retrieved during the biopsy procedure was higher for upgraded cases (mean 10 vs. 7), however, the mean size of the imaging target was not predictive of upgrade. The reason for this findings is not clear. No other clinicopathologic features at presentation on CNB were associated with upgrade at excision, as observed in other studies [22, 25], however, the presence of necrosis on CNBs showed a positive trend. Sullivan et al. found that upgrades were more common in younger patients and in LCIS with necrosis, but these associations were not statistically significant [18]. A recent study by Foschini et al. found that the extent of radiologic microcalcifications was associated with upgrade at excision [29].
We observed apocrine features in what would otherwise qualify as FLCIS. Apocrine features in LCIS were initially described in 1984 by Eusebi et al. in two cases, including one associated with invasive carcinoma [42]. The WHO 5th edition recognizes apocrine morphology only in PLCIS. We have observed that many practicing pathologists interpret apocrine morphology in LCIS as evidence of PLCIS, even in the absence of nuclear pleomorphism. For accurate classification, it is therefore important to recognize that apocrine features can also occur in FLCIS. It is unclear if these lesions have been previously classified as PLCIS based solely on the presence of apocrine features, or whether the presence of apocrine features were not recognized in FLCIS [43].
Morphologic subtypes of LCIS are characterized by biallelic CDH1 inactivation, which translates into loss or aberrant membranous E-cadherin expression by IHC, similar to CLCIS [44]. Recent genomic studies have shown that PLCIS and FLCIS represent more genetically complex lesions than CLCIS, with highly recurrent ERBB2 and ERBB3 gene alterations [35, 36]. In addition, genetic analysis of synchronous paired variant LCIS and ILC demonstrated similar or identical copy number alterations and mutational profile [35]. These findings support a more robust role as precursors of invasive carcinoma for FLCIS and PLCIS from compared to classic LCIS, in accordance with the WHO 5th classification scheme.
Although there appears to be no difference in prognosis between apocrine and non-apocrine PLCIS [21], array-based comparative genomic hybridization studies showed that apocrine PLCIS have significantly more genomic alterations than non-apocrine PLCIS, including amplification of cyclin D1 and HER2 genes [45]. In addition, apocrine PLCIS has been shown to be more frequently ER negative compared to non-apocrine PLCIS and CLCIS [43, 45]. Genomic analysis in a series of 20 FLCIS showed that these lesions share the same genetic complexity as apocrine PLCIS [43], with decreased expression of ER (23% vs. 100%) and more frequent HER2 overexpression (31% vs. 0%) compared to non-apocrine PLCIS. We also found that LCIS with apocrine features (PLCIS or FLCIS) was more frequently ER negative compared to non-apocrine variants (45% vs. 5%) and was more likely to be HER2 positive (30% vs. 5%). Further studies are needed to determine if apocrine FLCIS is genetically different from non-apocrine FLCIS.
Our results are in keeping with previous findings that PLCIS and FLCIS are frequently associated with invasive carcinoma or DCIS, and that these lesions should be not be managed as CLCIS [21]. Therefore, excision should be recommended, even if there is radiologic–pathologic concordance at presentation. At present there is no consensus regarding optimal margin clearance (we currently report margins for PLCIS and FLCIS at our institution, but not for LCIS-PF), adjuvant radiotherapy and the benefits of adjuvant hormonal therapy for PLCIS and FLCIS [46] and there are substantial variations in the classification and management of LCIS and its variants. In the latest AJCC Cancer Staging Manual 8th Ed. and in the NCCN 2020 guidelines LCIS is classified only as a high risk lesion, and there is no mention of LCIS variants [37, 38]. In contrast, the practice guidelines released by ESMO do not mention FLCIS, but recommend that PLCIS be managed as high-grade DCIS [39].
Information on the long term follow-up of patients with only FLCIS or PLCIS continues to be very limited. In our series, three patients without immediate upgrade at excision developed microinvasive or invasive carcinoma on follow-up and one of the patients died of disease.
Our study also demonstrates that additional workup to rule out (micro)invasion on CNB is warranted on PLCIS and FLCIS, especially in cases with extensive LCIS, presence of fibrosis, inflammation, or slight increase in stromal cellularity [47]. Deeper H&E levels and/or IHC, preferably a double immunostaining technique such as ADH5, or p120 catenin or a cytokerain used as sole marker, can help identify foci of (micro)invasion associated with FLCIS or PLCIS.
Limitations to our study include the small number of cases and its retrospective design with potential selection bias as not all patients with a diagnosis of LCIS other than classic were referred for excision at our institution, as well as inclusion of only cases with available material for review.
In summary, the upgrade rates of PLCIS and FLCIS at excision were 25 and 17%, respectively. These lesions frequently show an unfavorable biomaker profile with frequent HER2 overexpression. The findings of this study and of previously published series, support surgical management of PLCIS and FLCIS for complete histologic evaluation, given frequent association with (micro)invasive carcinoma, most of which are not detected radiologically. In addition, our study is the first one to describe apocrine features in FLCIS, only previously reported in PLCIS. While apocrine PLCIS appear be more genomically complex than non-apocrine PLCIS, the significance of apocrine features in FLCIS is unknown.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen YY, Decker T, King TA, Palacios J, Shin SJ, Simpson PT lobular carcinoma in situ. In: WHO classification of tumours editorial board. breast tumours. 5th ed. Lyon, France: IARC; 2019. p. 71–4
Dabbs DJ, Kaplai M, Chivukula M, Kanbour A, Kanbour-Shakir A, Carter GJ. The spectrum of morphomolecular abnormalities of the E-cadherin/catenin complex in pleomorphic lobular carcinoma of the breast. Appl Immunohistochem Mol Morphol. 2007;15:260–6.
Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941;17:491–5.
Page DL, Kidd TE Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22:1232–9.
King TA, Pilewskie M, Muhsen S, Patil S, Mautner SK, Park A, et al. Lobular carcinoma in situ: a 29-Year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol. 2015;33:3945–52.
Ansquer Y, Delaney S, Santulli P, Salomon L, Carbonne B, Salmon R. Risk of invasive breast cancer after lobular intra-epithelial neoplasia: review of the literature. Eur J Surg Oncol. 2010;36:604–9.
Murray MP, Luedtke C, Liberman L, Nehhozina T, Akram M, Brogi E. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer. 2013;119:1073–9.
Nakhlis F, Gilmore L, Gelman R, Bedrosian I, Ludwig K, Hwang ES, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in-situ in patients with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol. 2016;23:722–8.
Niell B, Specht M, Gerade B, Rafferty E. Is excisional biopsy required after a breast core biopsy yields lobular neoplasia? AJR Am J Roentgenol. 2012;199:929–35.
D’Alfonso TM, Wang K, Chiu YL, Shin SJ. Pathologic upgrade rates on subsequent excision when lobular carcinoma in situ is the primary diagnosis in the needle core biopsy with special attention to the radiographic target. Arch Pathol Lab Med. 2013;137:927–35.
Lakhani SR, Schnitt SJ, O’Malley F, van de Vijver MJ, Simpson PT, Palacios J Lobular neoplasia. In: Lakhani SR, Ellis I, Schnitt SJ, Tan PH, Van de Vijver MJ, editors. WHO Classification of Tumours of the Breast. Geneva, Switzerland: WHO Press; 2012. p. 78-80
Frost AR, Tsangaris TN, Silverberg SG. Pleomorphic lobular carcinoma in situ. AJSP: Rev Rep. 1996;1:27–31.
Middleton LP, Palacios DM, Bryant BR, Krebs P, Otis CN, Merino MJ. Pleomorphic lobular carcinoma: morphology, immunohistochemistry, and molecular analysis. Am J Surg Pathol. 2000;24:1650–6.
Fadare O, Dadmanesh F, Alvarado-Cabrero I, Snyder R, Stephen Mitchell J, Tot T, et al. Lobular intraepithelial neoplasia [lobular carcinoma in situ] with comedo-type necrosis: a clinicopathologic study of 18 cases. Am J Surg Pathol. 2006;30:1445–53.
Chivukula M, Haynik DM, Brufsky A, Carter G, Dabbs DJ. Pleomorphic lobular carcinoma in situ (PLCIS) on breast core needle biopsies: clinical significance and immunoprofile. Am J Surg Pathol. 2008;32:1721–6.
Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol. 2008;21:1208–16.
Carder PJ, Shaaban A, Alizadeh Y, Kumarasuwamy V, Liston JC, Sharma N. Screen-detected pleomorphic lobular carcinoma in situ (PLCIS): risk of concurrent invasive malignancy following a core biopsy diagnosis. Histopathology. 2010;57:472–8.
Sullivan ME, Khan SA, Sullu Y, Schiller C, Susnik B. Lobular carcinoma in situ variants in breast cores: potential for misdiagnosis, upgrade rates at surgical excision, and practical implications. Arch Pathol Lab Med. 2010;134:1024–8.
Flanagan MR, Rendi MH, Calhoun KE, Anderson BO, Javid SH. Pleomorphic lobular carcinoma in situ: radiologic-pathologic features and clinical management. Ann Surg Oncol. 2015;22:4263–9.
Susnik B, Day D, Abeln E, Bowman T, Krueger J, Swenson KK, et al. Surgical outcomes of lobular neoplasia diagnosed in core biopsy: prospective study of 316 cases. Clin Breast Cancer. 2016;16:507–13.
Shamir ER, Chen YY, Chu T, Pekmezci M, Rabban JT, Krings G. Pleomorphic and florid lobular carcinoma in situ variants of the breast: a clinicopathologic study of 85 cases with and without invasive carcinoma from a single academic center. Am J Surg Pathol. 2019;43:399–408.
Nakhlis F, Harrison BT, Giess CS, Lester SC, Hughes KS, Coopey SB, et al. Evaluating the rate of upgrade to invasive breast cancer and/or ductal carcinoma in situ following a core biopsy diagnosis of non-classic lobular carcinoma in situ. Ann Surg Oncol. 2019;26:55–61.
Fasola CE, Chen JJ, Jensen KC, Allison KH, Horst KC. Characteristics and clinical outcomes of pleomorphic lobular carcinoma in situ of the breast. Breast J. 2018;24:66–9.
Guo T, Wang Y, Shapiro N, Fineberg S. Pleomorphic lobular carcinoma in situ diagnosed by breast core biopsy: clinicopathologic features and correlation with subsequent excision. Clin Breast Cancer. 2018;18:e449–e54.
Savage JL, Jeffries DO, Noroozian M, Sabel MS, Jorns JM, Helvie MA. Pleomorphic lobular carcinoma in situ: imaging features, upgrade rate, and clinical outcomes. AJR Am J Roentgenol. 2018;211:462–7.
Masannat YA, Husain E, Roylance R, Heys SD, Carder PJ, Ali H, et al. Pleomorphic LCIS what do we know? A UK multicenter audit of pleomorphic lobular carcinoma in situ. Breast. 2018;38:120–4.
Desai AA, Jimenez RE, Hoskin TL, Day CN, Boughey JC, Hieken TJ. Treatment outcomes for pleomorphic lobular carcinoma in situ of the breast. Ann Surg Oncol. 2018;25:3064–8.
Hoffman DI, Zhang PJ, Tchou J. Breast-conserving surgery for pure non-classic lobular carcinoma in situ: a single institution’s experience. Surg Oncol. 2019;28:190–4.
Foschini MP, Miglio R, Fiore R, Baldovini C, Castellano I, Callagy G, et al. Pre-operative management of pleomorphic and florid lobular carcinoma in situ of the breast: Report of a large multi-institutional series and review of the literature. Eur J Surg Oncol. 2019;45:2279–86.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American society of clinical oncology/college of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–82.
Canas-Marques R, Schnitt SJ. E-cadherin immunohistochemistry in breast pathology: uses and pitfalls. Histopathology. 2016;68:57–69.
Dabbs DJ, Schnitt SJ, Geyer FC, Weigelt B, Baehner FL, Decker T, et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol. 2013;37:e1–11.
De Brot M, Koslow Mautner S, Muhsen S, Andrade VP, Mamtani A, Murray M, et al. Pleomorphic lobular carcinoma in situ of the breast: a single institution experience with clinical follow-up and centralized pathology review. Breast Cancer Res Treat. 2017;165:411–20.
Shamir ER, Chen YY, Krings G. Genetic analysis of pleomorphic and florid lobular carcinoma in situ variants: frequent ERBB2/ERBB3 alterations and clonal relationship to classic lobular carcinoma in situ and invasive lobular carcinoma. Mod Pathol. 2020;33:1078–91.
Harrison BT, Nakhlis F, Dillon DA, Soong TR, Garcia EP, Schnitt SJ, et al. Genomic profiling of pleomorphic and florid lobular carcinoma in situ reveals highly recurrent ERBB2 and ERRB3 alterations. Mod Pathol. 2020;33:1287–97.
Hortobagyi GN, Connolly JL, D’Orsi CJ, Edge SB, Mittendorf EA, Rugo HS, et al. Breast. In: The AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. p. 589-636
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Breast Cancer, [Internet], Version 6.2020 [Cited 24 November 2020]. Available from https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1674.
Blair SL, Emerson DK, Kulkarni S, Hwang ES, Malcarne V, Ollila DW. Breast surgeon’s survey: no consensus for surgical treatment of pleomorphic lobular carcinoma in situ. Breast J. 2013;19:116–8.
Khoury T, Karabakhtsian RG, Mattson D, Yan L, Syriac S, Habib F, et al. Pleomorphic lobular carcinoma in situ of the breast: clinicopathological review of 47 cases. Histopathology. 2014;64:981–93.
Eusebi V, Betts C, Haagensen DE Jr, Gugliotta P, Bussolati G, Azzopardi JG. Apocrine differentiation in lobular carcinoma of the breast: a morphologic, immunologic, and ultrastructural study. Hum Pathol. 1984;15:134–40.
Shin SJ, Lal A, De Vries S, Suzuki J, Roy R, Hwang ES, et al. Florid lobular carcinoma in situ: molecular profiling and comparison to classic lobular carcinoma in situ and pleomorphic lobular carcinoma in situ. Hum Pathol. 2013;44:1998–2009.
Wen HY, Brogi E. Lobular Carcinoma In Situ. Surg Pathol Clin. 2018;11:123–45.
Chen YY, Hwang ES, Roy R, DeVries S, Anderson J, Wa C, et al. Genetic and phenotypic characteristics of pleomorphic lobular carcinoma in situ of the breast. Am J Surg Pathol. 2009;33:1683–94.
Schnitt SJ, Brogi E, Chen YY, King TA, Lakhani SR. American registry of pathology expert opinions: the spectrum of lobular carcinoma in situ: diagnostic features and clinical implications. Ann Diagn Pathol. 2020;45:151481.
Ross DS, Hoda SA. Microinvasive (T1mic) lobular carcinoma of the breast: clinicopathologic profile of 16 cases. Am J Surg Pathol. 2011;35:750–6.
Funding
This work was supported in part by a Cancer Center Support Grant of the National Institute of Health/National Cancer Institute (grant number P30CA008748).
Author information
Authors and Affiliations
Contributions
MGK and EB performed study concept and design. MGK, MPM, KC, CC and EB developed methodology, acquisition and analysis of data. MGK performed statistical analysis. MGK, MPM, KC, CC, MM and EB performed writing, reviews and revision of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (Protocol # 17-287)
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuba, M.G., Murray, M.P., Coffey, K. et al. Morphologic subtypes of lobular carcinoma in situ diagnosed on core needle biopsy: clinicopathologic features and findings at follow-up excision. Mod Pathol 34, 1495–1506 (2021). https://doi.org/10.1038/s41379-021-00796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00796-9
This article is cited by
-
Immediate and delayed risk of breast cancer associated with classic lobular carcinoma in situ and its variants
Breast Cancer Research and Treatment (2024)
-
High-risk and selected benign breast lesions diagnosed on core needle biopsy: Evidence for and against immediate surgical excision
Modern Pathology (2022)
-
Current Perspectives on Lobular Neoplasia of the Breast
Current Radiology Reports (2022)
-
The morphologic spectrum of lobular carcinoma in situ (LCIS) observations on clinical significance, management implications and diagnostic pitfalls of classic, florid and pleomorphic LCIS
Virchows Archiv (2022)