Abstract
Proliferative fasciitis (PF) and proliferative myositis (PM) are rare benign soft tissue lesions, usually affecting the extremities of middle-aged or older adults. Presenting as poorly circumscribed masses, they histologically show bland spindle cell proliferation in a myxoid to fibrous background and a hallmark component of large epithelioid “ganglion-like” cells in various numbers, which may lead to their misdiagnosis as sarcoma. PF/PM has been long considered as reactive, akin to nodular fasciitis; however, its pathogenesis has remained unknown. In this study, we analyzed the FOS status in 6 PF/PMs (5 PFs and 1 PM). Five PF/PMs occurred in adults, all showing diffuse strong expression of c-FOS primarily in the epithelioid cells, whereas spindle cell components were largely negative. Using fluorescence in situ hybridization (FISH), all 5 c-FOS-immunopositive tumors showed evidence of FOS gene rearrangement in the epithelioid cells. RNA sequencing in 1 case detected a FOS-VIM fusion transcript, which was subsequently validated by reverse transcriptase-polymerase chain reaction, Sanger sequencing, and VIM FISH. The one pediatric PF case lacked c-FOS expression and FOS rearrangement. c-FOS immunohistochemistry was negative in 45 cases of selected mesenchymal tumor types with epithelioid components that may histologically mimic PF/PM, including pleomorphic sarcoma with epithelioid features and epithelioid sarcoma. Recurrent FOS rearrangement and c-FOS overexpression in PF/PM suggested these lesions to be neoplastic. FOS abnormality was largely restricted to the epithelioid cell population, clarifying the histological composition of at least 2 different cell types. c-FOS immunohistochemistry may serve as a useful adjunct to accurately distinguish PF/PM from mimics.
Similar content being viewed by others
Introduction
Proliferative fasciitis (PF) and proliferative myositis (PM) are rare benign soft tissue lesions. PM was first described formally by Kern in 1960 [1] and further characterized by Enzinger and Dulcey in 1967 [2]. Chung and Enzinger subsequently reported, in 1975, a subcutaneous counterpart of PM, known as PF [3]. PF and PM usually affect middle-aged and elderly adults with a roughly equal sex distribution [4]. Pediatric cases, though much rarer, have also been reported [5]. These tumors most commonly occur in the extremities, although trunk wall, head, and neck may also be involved. The tumors often present as rapidly growing masses, usually <5 cm in size, and may be associated with tenderness or pain. Microscopically, PF and PM are poorly circumscribed masses, composed of bland spindle cell proliferation in a myxoid to fibrous background and a hallmark component of large epithelioid “ganglion-like” cells in various numbers. Owing to the rapid growth, poor circumscription, and the presence of large epithelioid cells, PF/PM may be misdiagnosed as sarcoma [2, 3].
The pathogenesis of PF/PM remains unknown till date. Nonetheless, the lesions are widely considered as reactive, since they show self-limiting growth, scar-like histology, and a rare incidence of spontaneous involution [6]. Approximately 20% of PF/PM cases are associated with previous trauma [3]. These characteristics are so similar to those in the much more common nodular fasciitis that these lesions are often discussed together, or even considered as related entities. However, nodular fasciitis is now known to be a clonal disease, with USP6 gene rearrangement in up to 92% of cases (most commonly MYH9-USP6 fusion) [7, 8]; all PF/PMs tested thus far have lacked USP6 rearrangement [8,9,10], challenging the association. Reported cytogenetic abnormalities of PF/PM, including trisomy 2 in 2 cases [11, 12] and t(6;14)(q23;q32) in 1 case [13] have not been validated or characterized further.
Recently, we noted diffuse strong immunohistochemical expression of c-FOS in a case of PF. Guided by this unexpected finding, we analyzed archival PF/PM cases to determine whether FOS abnormality is recurrent in these lesions.
Materials and methods
Case selection
This study was approved by the Institutional Review Boards. Samples from 6 PF/PM cases identified in the institutional pathology archives (National Cancer Center Hospital, University of Tokyo Hospital, Komagome Hospital, and Kyorin University Hospital, all in Tokyo, Japan) were available for additional immunohistochemical and molecular analyses. All tumors were histologically reviewed by a soft-tissue pathologist (AY) and the diagnosis was confirmed. Clinical data were retrieved from medical records.
We also retrieved soft-tissue tumors with epithelioid cells that can enter the histological differential diagnosis of PF/PM. These included 15 pleomorphic sarcomas with epithelioid component (9 myxofibrosarcomas and 6 undifferentiated pleomorphic sarcomas), 10 epithelioid sarcomas (all deficient in SMARCB1 expression), 5 epithelioid hemangioendotheliomas (all immunopositive for CAMTA1), 5 pseudomyogenic hemangioendotheliomas (all immunopositive for FOSB), 5 embryonal rhabdomyosarcomas, and 5 tumors with ganglion cell component (2 ganglioneuromas and 3 ganglioneuroblastomas), although the latter 2 tumors types represent more historical than practical differentials. In addition, we collected 10 cases of nodular fasciitis (9 were tested positive for USP6 rearrangement).
Immunohistochemistry
Sections (4 μm-thick) from the formalin-fixed, paraffin-embedded (FFPE) block of each specimen were routinely deparaffinized. The sections were exposed to 3% hydrogen peroxide to block endogenous peroxidase activity. Heat-induced epitope retrieval was performed. The primary antibodies and staining conditions are summarized in Table 1. The slides were incubated for 1 h at room temperature, and subsequently labeled with the EnVision system (Dako, Glostrup, Denmark). c-FOS was stained in all cases, whereas FOSB, ERG, and SATB2 were stained for a subset of PF/PM cases when needed in the study process. Appropriate controls were used. For PF/PMs and other tumors with epithelioid cells, strong nuclear immunoreactivity in >50% of epithelioid cells was considered positive. For nodular fasciitis, the staining results were recorded descriptively, since it lacked epithelioid cells except a few scattered osteoclast-like giant cells.
Fluorescence in situ hybridization (FISH)
FISH was performed using 4-μm-thick FFPE sections from PF/PM specimens. We used break-apart probes to detect gene rearrangement of FOS, VIM, and FOSB (all obtained from GSP Lab, Inc., Kobe, Japan). FISH images were captured using the Metafer Slide Scanning Platform (MetaSystems, Altlussheim, Germany). Samples in which more than 20% of the epithelioid cells showed break-apart signals were considered positive for rearrangement. Particular care was taken to differentiate epithelioid cell nuclei from surrounding spindle cell or endothelial nuclei, with a close correlation with H&E and c-FOS-immunostained slides. Relevant positive and negative control cases were used for each probe.
RNA sequencing
FFPE sample sections from case 1 were deparaffinized and subjected to RNA extraction using an RNeasy FFPE kit (Qiagen, Hilden, Germany). RNA sequencing was performed using the TruSight Pan-Cancer panel (Illumina, San Diego, CA, USA), which targets 1385 cancer-related genes, following the manufacturer’s instruction. Sequencing was performed on a MiSeq instrument (Illumina) using MiSeq Reagent Kit v3 (Illumina) with 150 cycles. Fusion gene identification was performed using the RNA-Seq alignment applications STAR and Top-Hat2 (Illumina).
Reverse transcriptase-polymerase chain reaction (RT-PCR) and Sanger sequencing
Total RNA was extracted from the FFPE sample of case 1 using RecoverAll Total Nucleic Acid Isolation Kit (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from total RNA using Superscript III (Invitrogen). Tumor cDNA was subjected to PCR using the primer pairs 5′-GCTTCCCTTGATCTGACTGG-3′ and 5′-TCTCCAAAGGCTGCAGAAGT-3′ (predicted product size of 130 bp) to specifically amplify the fusion transcript. The PCR product was cleaned up using IllustraMicroSpin Columns (GE Healthcare, Chalfont St. Giles, UK). The isolated PCR product was then subjected to Sanger sequencing reaction using the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and analyzed on an ABI PRISM 3130xl Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The sequence chromatogram files were examined using FinchTV (version 1.4.0; Digital World Biology, Seattle, WA, USA).
Results
Clinicopathological findings
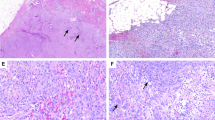
Clinicopathological findings of PF/PM cases are summarized in Table 2. All 6 patients were men. Five patients were adults (60–78 years old), and 1 was a child (1 year old). The lesions were located in the arm (n = 2), forearm (n = 1), shoulder (n = 1), thigh (n = 1), and groin (n = 1). Five tumors were subcutaneous (PF), whereas 1 was intramuscular (PM). The lesions measured 7–30 mm (median, 20 mm). The tumor was resected in 4 cases, and expectantly observed after needle biopsy in 2 cases. Follow-up was available for 4 patients, and none had evidence of disease over 8 to 51 months. One tumor (case 4), which received needle biopsy only, involuted and was undetectable at 8 months. Histologically, all adult tumors were poorly circumscribed with variable infiltration into the surrounding adipose tissue (Fig. 1) or the skeletal muscle. The lesions consisted of fascicular proliferation of non-atypical long spindle cells in a fibro-edematous background, in which characteristic large epithelioid cells were admixed (Figs. 2a, c, e, g, 4a, 5a). The latter harbored abundant amphophilic cytoplasm, vesicular nuclei, and prominent nucleoli, similar to ganglion cells, and demonstrated no or focally mild nuclear pleomorphism. The number and distribution of epithelioid cells were variable, ranging from isolated and scattered to dense in adult cases. In resected specimens, the epithelioid cells were abundant in the center of the lesions, while they were scattered amongst predominant spindle cells in the periphery. In the only pediatric case, numerous epithelioid cells formed diffuse sheets, without accompanying spindle cell proliferation, in a well-circumscribed tumor (Fig. 7a), which was typical for this age group [5]. Overall, all 6 cases showed classic clinicopathological findings of PF/PM.
a, c Characteristic large epithelioid cells densely proliferate, particularly in the center of the lesion. These cells harbor vesicular chromatin, prominent nucleoli, and amphophilic cytoplasm, similar to ganglion cells (A, case 1; C, case 2). Inset in (a) shows a magnified view of a ganglion-like cell. b, d c-FOS immunohistochemistry (in the areas depicted in (a) and (c), respectively) shows diffuse strong expression in epithelioid cells. e, g In areas, particularly at the periphery of the lesion, fascicular proliferation of bland spindle cells predominates, with only a few scattered epithelioid cells admixed within (E, case 1, G, case 4). f, h c-FOS immunohistochemistry in these areas (depicted in (e) and (g), respectively) identifies a few scattered epithelioid cells, whereas spindle cell component is virtually negative for c-FOS expression.
c-FOS overexpression and FOS rearrangement in adult PF/PM
The results are summarized in Table 2. Immunohistochemically, all 5 PF/PM tumors in adults showed c-FOS expression (Figs. 2b, d, f, h,4b, 5b). The staining diffusely decorated the large epithelioid cells and the reactivity was so intense that, in addition to nuclear labeling, the cytoplasm also exhibited weaker staining. Similarly labeled smaller epithelioid cells, small round cells, and spindle cells were also observed in the tumor tissues in a much smaller number, some of which likely represented the truncated large epithelioid cells. In contrast, spindle cell components were mostly negative for c-FOS. Three of these cases (cases 1, 2, and 4) were also stained with FOSB antibody and showed negative to weak focal staining in spindle cells only.
Based on FISH, all the 5 c-FOS-immunopositive PF/PMs showed evidence of FOS gene rearrangement in the epithelioid cells, whereas spindle cell component was largely negative for FOS rearrangement (Figs. 3, 4c, 5c). A low-level FOS copy number increase was observed in some epithelioid cells with gene rearrangement.
a The tumor consists of large epithelioid cells with mild pleomorphism and spindle cell proliferation. b The epithelioid cells are immunohistochemically positive for c-FOS, whereas spindle cells are negative. c The epithelioid cells harbor FOS gene rearrangement as detected by FISH (arrows indicate split 3′ and 5′ probes), as well as a low-level copy number increase.
One FOS-rearranged PF (case 1) had adequate material for targeted RNA sequencing, which detected a FOS-VIM fusion transcript. The fusion was validated using RT-PCR and Sanger sequencing. The breakpoint in FOS was located at c.724 in exon 4, which was fused to the reverse complementary strand of exon 1 in VIM (Fig. 6a). The predicted protein lost 138 amino acids from the C-terminus of wild-type c-FOS protein and gained additional 10 amino acids at the C-terminal end before the stop codon, maintaining the leucine zipper domain but losing the transactivation domain of c-FOS protein (Fig. 6b). The fusion was further validated using FISH with VIM break-apart probe (Fig. 6c). VIM break-apart FISH was performed for the remaining 4 FOS-rearranged PF/PM tumors; however, none showed evidence of VIM rearrangement.
a RNA sequencing detected a FOS-VIM fusion transcript, which was validated by RT-PCR and Sanger sequencing. The breakpoint in FOS was located at c.724 in the middle of exon 4, which was fused to the reverse complementary strand of exon 1 of VIM. b The predicted protein lost 138 C-terminal amino acids (AA) of wild-type c-FOS protein and gained additional 10 AA at C-terminal end before the stop codon, which maintains the leucine zipper domain (LZD) but loses the transactivation domain (TAD) of c-FOS protein. c The presence of FOS-VIM fusion was also validated by VIM rearrangement as detected by a VIM break-apart FISH assay (arrows indicate split 3′ and 5′ probes).
Lack of c-FOS overexpression and FOS rearrangement in the pediatric PF case
The only pediatric case of PF studied (case 3) lacked both c-FOS immunoexpression and FOS rearrangement (Fig. 7b,c). Both FOSB immunohistochemistry and FOSB FISH were also negative.
No evidence of endothelial or osteoblastic differentiation in PF/PM
To examine whether FOS rearrangement in PF/PM might result in similar lines of differentiation to epithelioid hemangioma or osteoid osteoma/osteoblastoma, we stained 4 FOS-rearranged PF/PM tumors (cases 1, 2, 4, and 5) using ERG and SATB2 antibodies, which are sensitive vascular endothelial and osteoblastic markers, respectively. All 4 PF/PMs tested were negative for ERG. SATB2 was weakly and focally expressed in epithelioid cells in 2 of the 4 cases, while the remaining 2 showed negative results.
Utility of c-FOS immunohistochemistry in differential diagnosis
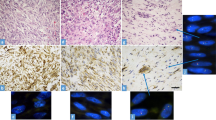
We performed c-FOS immunohistochemistry in histological mimics of PF/PM to test its diagnostic utility. The results are presented in Table 3 and illustrated in Fig. 8. None of the 45 mesenchymal tumors with epithelioid components showed strong nuclear staining in >50% of cells, although heterogeneous weak to strong immunoreactive cells were focally observed in many cases. All 10 cases of nodular fasciitis contained a small number of spindle or small rounded cells that were weakly to strongly positive for c-FOS in up to 10% of cells.
a, b An undifferentiated pleomorphic sarcoma with epithelioid features focally shows heterogeneous weak to strong c-FOS immunoreactivity. c, d An epithelioid sarcoma focally shows heterogeneous weak to moderate c-FOS immunoreactivity. e, f A case of nodular fasciitis containing a small number of spindled or small rounded cells that weakly to moderately expressed c-FOS.
Discussion
PF and PM are rare benign soft tissue lesions with distinct clinical and histological features, and yet unknown pathogenesis. In this study, we identified recurrent FOS rearrangement in archival PF/PM cases with classic clinicopathological features. Among a total of 6 PF/PMs, 5 adult tumors showed FOS rearrangement as per FISH, along with diffuse and strong c-FOS positivity by immunohistochemistry. One of these cases, analyzed by RNA sequencing, showed a FOS-VIM fusion transcript, which was validated by RT-PCR, Sanger sequencing, and VIM FISH. Although the karyotype t(6;14)(q23;q32) was previously identified in a case of PM through conventional G-banding 13, the FOS locus 14q24 was not involved in it.
FOS gene encodes a leucine zipper protein c-FOS, which dimerizes with the JUN family protein and forms the transcription factor complex AP-1 [14]. FOS physiologically plays important roles in cell growth, differentiation, and survival. Oncogenic c-FOS activation via FOS gene rearrangement has been reported in epithelioid hemangioma [15, 16] and osteoid osteoma/osteoblastoma [17], in which FOS fuses with diverse partners, including LMNA, VIM, MBNL1, and lincRNA (RP11-326N17.1) in epithelioid hemangioma [15, 16], and ANKH, KIAA1199, MYO1B, and intergenic non-coding regions in osteoid osteoma/osteoblastoma [17]. In the reported cases, FOS gene is invariably rearranged within exon 4 and fused to various protein-coding or non-coding regions, forming a stop codon at or immediately after the breakpoint. Truncated c-FOS protein maintains the leucine zipper domain while losing the transactivating domain and C-terminal regulatory sequences, thus hampering normal rapid mRNA and protein degradation and leading to abnormally stabilized mutant c-FOS expression [17, 18]. The causal relationship between FOS rearrangement and epithelioid hemangioma was supported by an in vitro experiment in which ectopic expression of truncated c-FOS stimulated endothelial sprouting via upregulation of matrix metalloproteinases and components of the Notch signaling pathway [18]. The fusion structure of FOS-VIM in our case 1 was similar to that reported in epithelioid hemangioma [16], which, along with diffuse strong c-FOS immunoexpression, suggests a similar c-FOS activating mechanism in PF and PM. However, further studies are required to understand the detailed pathogenesis.
PF and PM have long been considered as reactive, owing to self-limiting growth, scar-like microscopic appearance, association with trauma in a subset, and rare spontaneous involution [1,2,3, 6]. The demonstration of recurrent FOS rearrangement in PF/PM challenges this notion and suggested a neoplastic nature of this disorder, adding to the growing list of “pseudotumors” that have been re-interpreted as neoplasms after the discovery of recurrent genetic abnormalities (most notably, the frequent USP6 rearrangement in nodular fasciitis [7, 8] and myositis ossificans [19]). Spontaneous regression, which is rarely documented and observed in one of our cases after needle biopsy, may indicate PF/PM to possibly represent another example of “transient neoplasia”, a concept originally proposed for nodular fasciitis [7].
c-FOS immunoreactivity and FOS rearrangement were primarily observed in epithelioid cells, a hallmark of PF/PM, but they were mostly absent in the accompanying spindle cells. The nature of epithelioid cells in PF/PM has been a subject of debate, with proposed hypotheses including myogenic, fibroblastic, myofibroblastic, and histiocytic lineages [20]. Electron microscopic evidence have suggested these cells to be modified fibroblasts, since they harbor abundant rough endoplasmic reticulum, thin filament of actin type, along with intracytoplasmic collagen production [3, 20, 21]. Immunohistochemically, epithelioid cells have been positive for vimentin, and negative or focally positive for smooth muscle actin [21]. Enzinger et al. had speculated epithelioid and spindle cells to possibly form a spectrum, since they observed an intermediate form between these cells [2, 3]. However, the existence of intermediate cells was not confirmed by a subsequent ultrastructural study by Lundgren et al [21]. Our study suggested the epithelioid and spindle cells to be distinct populations, with the only former likely representing a clonal element, admixed with reactive spindle cells. Nonetheless, a much smaller number of small round cells and spindle cells that expressed c-FOS were also admixed, and their nature and relationship to large epithelioid cells remain unclear.
The heterogeneous histological composition of PF/PM is reminiscent of osteoid osteoma, in which FOS fusion is restricted to the osteoblastic cells that line immature bone and osteoid within conspicuous granulation-like reactive tissue. PF/PM is reportedly associated with bone formation in rare instances [1, 2, 13], leading to an early hypothesis that it might represent a precursor to myositis ossificans [1]. Later studies had also repeatedly suggested epithelioid cells in PF/PM to show some resemblance with differentiating osteoblasts, both at the light and electron microscopic levels [2, 3, 21]. However, none of the epithelioid cells in the 4 FOS-rearranged PF/PMs tested in this study showed diffuse strong SATB2 expression, unlike osteoid osteoma/osteoblastoma [22], thus not supporting the immediate phenotypic link between these distinctive diseases in soft tissues and bone.
Both FOS rearrangement and c-FOS immunoreactivity were negative in the only pediatric PF sample examined in this study. Pediatric PF/PM is an exceedingly rare lesion, with some recognized differences from adult counterparts, including good circumscription, lobulation, diffuse sheet of epithelioid cells, and lack of spindle cell component [5]. This pediatric tumor also lacked FOSB expression or gene rearrangement, although FOSB fusion has been reported as an alternative oncogenic mechanism in epithelioid hemangioma or osteoblastoma that lack FOS alterations [17, 23]. Interestingly, a previous study identified diffuse strong FOSB immunoexpression in a small subset of PF [24]. As the present study is limited by the small number of cases, future studies on a larger cohort are necessary to establish the prevalence of FOS rearrangement, and to determine whether alternative FOSB rearrangement may exist in a subset and whether pediatric PF/PM shares pathogenesis with adult cases.
c-FOS immunohistochemistry can be diagnostically helpful. Unlike PF/PM, none of the 45 mimicking tumors with epithelioid component showed diffuse (>50%) strong c-FOS expression. In bone, c-FOS staining has been proposed as an ancillary tool for distinguishing osteoid osteoma/osteoblastoma from osteosarcoma [25, 26]. Nodular fasciitis invariably contained a small number of scattered spindled or small rounded cells that were weakly to strongly stained for c-FOS. Although this low-power appearance may superficially resemble that of PF/PM, the c-FOS-positive cells in nodular fasciitis do not possess large epithelioid cytology, and the staining is heterogeneous unlike uniform strong labeling in PF/PM.
In summary, we demonstrated that PF/PM harbors recurrent FOS rearrangement and c-FOS overexpression, thus suggesting these lesions as neoplasms. FOS abnormality was largely restricted to the epithelioid cell population, clarifying the histological composition of at least 2 different cell types. c-FOS immunohistochemistry may serve as a useful adjunct to accurately distinguish PF/PM from mesenchymal mimics with epithelioid elements.
References
Kern WH. Proliferative myositis; a pseudosarcomatous reaction to injury: a report of seven cases. Arch Pathol. 1960;69:209–16.
Enzinger FM, Dulcey F. Proliferative myositis. Report of thirty-three cases. Cancer. 1967;20:2213–23.
Chung EB, Enzinger FM. Proliferative fasciitis. Cancer. 1975;36:1450–8.
Wang WL, Lazar AJ. Proliferative fasciitis and proliferative myositis. In: the WHO Classification of Tumours Editorial Board (ed). WHO Classificatin of Tumours. Soft Tissue and Bone Tumours., 5th ed., IARC: Lyon, 2020, pp 51-52.
Meis JM, Enzinger FM. Proliferative fasciitis and myositis of childhood. Am J Surg Pathol. 1992;16:364–72.
Kato K, Ehara S, Nishida J, Satoh T. Rapid involution of proliferative fasciitis. Skelet Radio. 2004;33:300–2.
Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427–33.
Oliveira AM, Chou MM. USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol. 2014;45:1–11.
Erber R, Agaimy A. Misses and near misses in diagnosing nodular fasciitis and morphologically related reactive myofibroblastic proliferations: experience of a referral center with emphasis on frequency of USP6 gene rearrangements. Virchows Arch. 2018;473:351–60.
Shin C, Low I, Ng D, Oei P, Miles C, Symmans P. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784–91.
Dembinski A, Bridge JA, Neff JR, Berger C, Sandberg AA. Trisomy 2 in proliferative fasciitis. Cancer Genet Cytogenet. 1992;60:27–30.
Ohjimi Y, Iwasaki H, Ishiguro M, Isayama T, Kaneko Y. Trisomy 2 found in proliferative myositis cultured cell. Cancer Genet Cytogenet. 1994;76:157.
McComb EN, Neff JR, Johansson SL, Nelson M, Bridge JA. Chromosomal anomalies in a case of proliferative myositis. Cancer Genet Cytogenet. 1997;98:142–4.
Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–73.
Huang SC, Zhang L, Sung YS, Chen CL, Krausz T, Dickson BC, et al. Frequent FOS gene rearrangements in epithelioid hemangioma: a molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol. 2015;39:1313–21.
van IJzendoorn DG, de Jong D, Romagosa C, Picci P, Benassi MS, Gambarotti M, et al. Fusion events lead to truncation of FOS in epithelioid hemangioma of bone. Genes Chromosomes Cancer. 2015;54:565–74.
Fittall MW, Mifsud W, Pillay N, Ye H, Strobl AC, Verfaillie A, et al. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun. 2018;9:2150.
van IJzendoorn DGP, Forghany Z, Liebelt F, Vertegaal AC, Jochemsen AG, Bovee JVMG, et al. Functional analyses of a human vascular tumor FOS variant identify a novel degradation mechanism and a link to tumorigenesis. J Biol Chem. 2017;292:21282–90.
Bekers EM, Eijkelenboom A, Grunberg K, Roverts RC, de Rooy JWJ, van der Geest ICM, et al. Myositis ossificans - Another condition with USP6 rearrangement, providing evidence of a relationship with nodular fasciitis and aneurysmal bone cyst. Ann Diagn Pathol. 2018;34:56–59.
Ushigome S, Takakuwa T, Takagi M, Koizumi H, Morikubo M. Proliferative myositis and fasciitis. Report of five cases with an ultrastructural and immunohistochemical study. Acta Pathol Jpn. 1986;36:963–71.
Lundgren L, Kindblom LG, Willems J, Falkmer U, Angervall L. Proliferative myositis and fasciitis. A light and electron microscopic, cytologic, DNA-cytometric and immunohistochemical study. APMIS. 1992;100:437–48.
Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63:36–49.
Antonescu CR, Chen HW, Zhang L, Sung YS, Panicek D, Agaram NP, et al. ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer. 2014;53:951–9.
Hung YP, Fletcher CD, Hornick JL. FOSB is a useful diagnostic marker for pseudomyogenic hemangioendothelioma. Am J Surg Pathol. 2017;41:596–606.
Amary F, Markert E, Berisha F, Ye H, Gerrand C, Cool P, et al. FOS expression in osteoid osteoma and osteoblastoma: a valuable ancillary diagnostic tool. Am J Surg Pathol. 2019;43:1661–7.
Lam SW, Cleven AHG, Kroon HM, Briaire-de Bruijn IH, Szuhai K, JVMG Bovée. Utility of FOS as diagnostic marker for osteoid osteoma and osteoblastoma. Virchows Arch. 2020;476:455–63.
Acknowledgements
We acknowledge Sachiko Miura, Toshiko Sakaguchi, Chizu Kina, Eijitsu Ryo, Hiroki Kakishima, Kaori Yamaguchi, Mei Fukuhara, Hiroshi Chigira, Kazuhiro Yoshida, Kei Sakuma, Kimiko Takeshita, and Minato Murata for superb technical assistance. We thank Dr. Suguru Fukushima for his help with sample collection in one case.
Funding
This work was supported in part by National Cancer Center Research and Development Fund 30-A-2 (A. Yoshida), and JSPS Grant-in-Aid for Young Scientists 18K15108 (A. Yoshida) and 20K16167 (N. Makise).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makise, N., Mori, T., Motoi, T. et al. Recurrent FOS rearrangement in proliferative fasciitis/proliferative myositis. Mod Pathol 34, 942–950 (2021). https://doi.org/10.1038/s41379-020-00725-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-00725-2