Abstract
Raising photoelectric conversion efficiency and enhancing heat management are two critical concerns for silicon-based solar cells. In this work, efficient Yb3+ infrared emissions from both quantum cutting and upconversion were demonstrated by adjusting Er3+ and Yb3+ concentrations, and thermo-manage-applicable temperature sensing based on the luminescence intensity ratio of two super-low thermal quenching levels was discovered in an Er3+/Yb3+ co-doped tungstate system. The quantum cutting mechanism was clearly decrypted as a two-step energy transfer process from Er3+ to Yb3+. The two-step energy transfer efficiencies, the radiative and nonradiative transition rates of all interested 4 f levels of Er3+ in NaY(WO4)2 were confirmed in the framework of Föster-Dexter theory, Judd-Ofelt theory, and energy gap law, and based on these obtained efficiencies and rates the quantum cutting efficiency was furthermore determined to be as high as 173% in NaY(WO4)2: 5 mol% Er3+/50 mol% Yb3+ sample. Strong and nearly pure infrared upconversion emission of Yb3+ under 1550 nm excitation was achieved in Er3+/Yb3+ co-doped NaY(WO4)2 by adjusting Yb3+ doping concentrations. The Yb3+ induced infrared upconversion emission enhancement was attributed to the efficient energy transfer 4I11/2 (Er3+) + 2F7/2 (Yb3+) → 4I15/2 (Er3+) + 2F5/2 (Yb3+) and large nonradiative relaxation rate of 4I9/2. Analysis on the temperature sensing indicated that the NaY(WO4)2:Er3+/Yb3+ serves well the solar cells as thermos-managing material. Moreover, it was confirmed that the fluorescence thermal quenching of 2H11/2/4S3/2 was caused by the nonradiative relaxation of 4S3/2. All the obtained results suggest that NaY(WO4)2:Er3+/Yb3+ is an excellent material for silicon-based solar cells to improve photoelectric conversion efficiency and thermal management.
Similar content being viewed by others
Introduction
The total amount of widely used fossil fuels on Earth is limited and decreases at an accelerating rate day by day owing to the increase of world population and the expansion of industrial scale1,2,3,4. Moreover, the continuous use of fossil fuels will result in worldwide energy depletion, serious environmental pollution, and greenhouse gas emissions. Therefore, an urgent task is to find clean and renewable energies to replace fossil fuels5,6,7. Amongst all renewable energies, including wind energy, tidal energy, nuclear energy, hydro-energy, and solar energy, the solar energy is highly favored. Consequently, in recent years, solar cells that can convert light energy into electrical energy have attracted growing interest, and many kinds of solar cells, such as silicon solar cells, dye-sensitized solar cells, cadmium telluride thin film solar cells, perovskite solar cells, quantum dots solar cells, and organic solar cells, have been developed8,9,10,11. Amongst all of these solar cells, the silicon-based solar cells are the most technically mature and well-commercialized. Usually, it is acceptable in practical applications if the photoelectric conversion efficiency of silicon-based solar cells is larger than 15%. In fact, the efficiency of 15% is just close to the half of its theoretical value of the silicon-based solar cells, and even 1% increase in efficiency is beneficial to the economic profits and promoting applications. Increasing the efficiency through technical improvement for the traditional silicon-based solar cells will greatly increase the economic expenses and lower the cost performance and that is not preferred.
Except for the technical improvement of the traditional solar cell processes, another route to raising the silicon-based solar cell efficiency is to introduce light conversion materials. One of the light conversion materials is capable of converting one photon with energy close to or higher than the two times silicon bandgap energy into two or more photons with energy larger than the silicon bandgap energy. This conversion is widely known as quantum cutting or down-conversion12,13,14,15. In the silicon-based solar cells, the photocurrent generated by quantum-cut photons can double in comparison with that before quantum cutting, thus further elevating the energy yield of the solar cells. Another type of light conversion materials for silicon-based solar cells is so-called upconversion materials, which can combine two or more infrared photons with single-photon energy lower than silicon bandgap energy to one photon with energy higher than the silicon bandgap energy16,17,18,19. This upconversion can expand the effective wavelength range of silicon-based solar cells, thus further elevating the energy yield of the solar cells. It should be mentioned that the use of either quantum cutting materials or upconversion materials can increase the electric energy yield of the silicon-based solar cells with no need of changing any performance of the silicon-based solar cells.
The photovoltaic devices must operate when they are exposed to sunlight. Especially, the concentrator solar cells suffer from high-intensity sunlight irradiation20,21,22,23. The high-intensity sunlight irradiation can result in a high temperature of the solar cells. At the high temperature, the photoelectric conversion efficiency will decrease since the electron and hole recombination efficiency will increase. Therefore, the thermal management for the solar cells cannot be disregarded. The advanced thermal management asks for the temperature measurements. Nowadays, the temperature can be detected via many techniques, for example, thermistor temperature measurement, thermocouple temperature measurement, infrared radiation temperature measurement, fluorescence temperature measurement and so on24,25,26. For the practical application of temperature measurement in solar cells, the technical limit, economic cost, and longevity must be taken into account. Amongst all these temperature measurement routes mentioned above, the fluorescence temperature measurement may be a better choice since the inorganic fluorescence materials can be easily injected into the silicon-based solar cells in a close contact way, and the fluorescence signal for detecting temperature can be read out in a contactless way. In addition, the inorganic fluorescence materials are chemically and physically stable. Therefore, finding a suitable fluorescence-based temperature-measuring material is a key step to advanced thermal management for solar cells.
In recent years, aiming at improving the performance of silicon-based solar cells, both quantum cutting and upconversion materials are widely studied27,28,29,30,31,32,33,34,35. Though the temperature sensing rooting in the spectral measurements is widely reported36,37,38, there are no attempts to use the fluorescence temperature measurement to realize thermal management for solar cells. To our best knowledge, each research paper is concentrated on one issue amongst quantum cutting, upconversion, and temperature sensing. In this work, we attempt to develop a material that integrates quantum cutting, upconversion, and temperature sensing into one together. To this end, the NaY(WO4)2 is chosen as host for the quantum cutting study of Er3+ and Yb3+ duo since it has moderate phonon energy, which enables effective nonradiative relaxations from the upper energy level to the lower quantum cutting level (responsible for the first step of quantum cutting) and relatively weak nonradiative transition of the lower quantum cutting level. Moreover, the rare earth ions in NaY(WO4)2 usually exhibit large transition rates39,40,41. Meanwhile, the WO42- group containing 5 ions can effectively space out the rare earth luminescence centers in NaY(WO4)2 host and further repress the depopulation effect of the quantum cutting levels via cross-relaxation between rare earth ions and self-generated fluorescence quenching42, thus benefiting the quantum cutting emission at high doping concentration. Moreover, Y3+ inclusion in NaY(WO4)2 host is beneficial to the high concentration doping of rare earth luminescence centers. Er3+ is chosen as the donor ion since it has uniformly distributed energy level structure in the energy space that may be helpful to the multistep energy transfers from donor to acceptor. Yb3+ is chosen as the acceptor ion since it has simplest energy level structure and suitable emission wavelength43,44.
In this work, the optimum Er3+ concentration was confirmed by taking the visible fluorescence quenching into account, and then the concentration-varied Yb3+ were introduced into the Er3+ concentration-optimized samples for the quantum cutting investigations. The quantum cutting mechanism was discovered by the optical spectroscopic analyses, and the quantum cutting efficiencies were calculated in assistance of Judd-Ofelt theory, Föster-Dexter theory, energy gap law. The nearly pure color upconversion emission of Yb3+ was observed under 1550 nm excitation, and the upconversion mechanism was conducted. Moreover, the temperature sensing based on the fluorescence intensity ratio was studied, and the thermal quenching mechanism of 4S3/2 green emission was assigned to nonradiative transition. All these results indicate that NaY(WO4)2:Er3+/Yb3+ phosphor integrating quantum cutting, upconversion, and temperature sensing in one system is an excellent material especially for the silicon-based concentrating solar cells in which it can be coated on the reflector side surface to realize the light conversions45.
Experimental section
Sample preparation
Tungstate phosphors NaY(1-x)Erx(WO4)2 (x = 0.5, 1.0, 2.0, 5.0, 10.0, 20.0 and 50.0) and NaY(0.95-y)Er0.5Yby(WO4)2 (y = 0.5, 1.0, 2.0, 5.0, 10.0, 20.0 and 50.0) were prepared via a high-temperature solid-state reaction method. The raw materials, Y2O3 (99.99%), Er2O3 (99.99%), and Yb2O3 (99.99%) were supplied by Shanghai Second Chemical Reagent Factory (China). Other chemicals including Na2CO3 and WO3 were obtained from Tianjin Reagent Chemicals Co Ltd. (China). All the chemicals were analytical grade and no further purification was carried out.
To prepare the samples, the starting materials were weighed according to the designed stoichiometric ratio. Then, the raw materials were ground in an agate mortar for 30 min to mix them evenly. The well-mixed batch was put into an alumina crucible, and then was placed into an electric furnace. After calcined in air at 1000°C for 4 hours, the sample was obtained when the electric furnace was gradually cooled down to room temperature.
Sample characterization
The crystal phase structure of the prepared samples was checked by an X-ray powder diffractometer (Shimadzu XRD-6000 equipped with Cu Kα1 radiation resource (λ = 0.15406 nm), in a 2θ range from 20° to 70° at a scanning step of 0.02 °/s. Hitachi F-4600 fluorescent spectrometer equipped with an internal 150 W xenon lamp was used to collect the visible luminescence spectra. The infrared luminescence spectra and the fluorescence lifetimes were measured by Edinburgh FLS1000 spectrofluorometer equipped with a sample holder for powders. The UC emission spectra were measured on a Hamamatsu Vis/IR mini- spectrophotometer C10083MD, and an external 1550 nm fiber output laser was conducted as the excitation source. The diffuse-reflection spectra of the samples were measured by a spectrophotometer UV-3600 (Shimadzu, Japan) equipped with an integrating sphere accessory (Ante, China, 206-23851-91). It should be mentioned that the BaSO4 powder provided by the spectrometer manufacturer was used as the reference for measuring the diffuse-reflection spectra. A homemade temperature controlling system DMU-450 with a temperature accuracy of better than 0.5 °C was used to control the sample temperature.
Results and discussion
Crystal structure
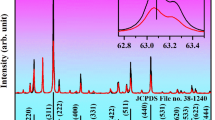
To identify the crystal structure of the obtained samples, the X-ray diffraction (XRD) patterns for all obtained NaY(WO4)2 phosphors were measured and are shown in Fig. S1 in the supporting information file. It is seen from Fig. S1 that the diffraction patterns of all synthesized samples are in good agreement with the diffraction pattern reported in the JCPDS card No. 48-0886, thus indicating all prepared samples are tetragonal-phased NaY(WO4)2 polycrystalline powders.
Quantum cutting of Er3+/Yb3+ co-doped NaY(WO4)2
This section primarily concentrates on the quantum cutting properties of Er3+/Yb3+ co-doped NaY(WO4)2 phosphors, including the observation of short wavelength absorption, optimization of the doping concentration, discovery of the quantum cutting mechanisms, and calculations of the quantum cutting efficiencies.
Short wavelength absorption of Er3+ in NaY(WO4)2
To examine the short wavelength absorption of Er3+ in NaY(WO4)2 phosphors, the excitation spectra for the samples with different Er3+ concentrations were measured by monitoring 552 nm emission corresponding to 4S3/2→4I15/2 transition and are shown in Fig. 1. It is seen that each spectrum contains five narrow absorption peaks and one broad absorption band. As marked in Fig. 1 the five narrow absorption peaks can be assigned to the transitions from 4I15/2 to 4F7/2, 4F5/2/4F3/2, 2H9/2, 4G11/2, and 4G7/2/2K15/2/4G9/2, respectively, but the broad absorption band corresponds to the W6+-O2- charge transfer transition. From Fig. 1 it is seen that the NaY(WO4)2:Er3+ exhibits plentiful narrow absorption bands in the range from 350 to 500 nm, and the most intense absorption transition locates at 378 nm corresponding to 4I15/2→4G11/2 transition.
Er3+ concentration optimization
It is known that intense absorption of Er3+ for the shorter wavelength will benefit the quantum cutting. The intense absorption asks for a high doping concentration. However, as common sense that the high doping concentration will result in the depopulation of the upper levels via cross relaxations which further depresses the quantum cutting efficiency. Therefore, optimization of the Er3+ doping concentration is required.
To examine the influence of Er3+ concentration on the population of upper metastable state, the emission spectra for all NaY(WO4)2:Er3+ phosphors were measured under 378 nm excitation and are shown in Fig. 2a. It was found that two intense emissions peaking at 530 and 552 nm corresponding to 2H11/2 → 4115/2 and 4S3/2 → 4115/2 transitions are observed. Two very weak emissions peaking at 657 and 700 nm corresponding 4F9/2 → 4115/2 and 2H9/2 → 4I9/2 transitions are observed when the spectra ranging from 600 to 750 nm were replotted as in the inset of Fig. 2a. This result also implies that all the levels locating between 4G11/2 and 2H11/2 depopulate through cascade nonradiative relaxations since their emissions of these levels are not observed even for the samples with low Er3+ concentrations. It is well known that the emission intensity of a level is proportional to its population, therefore, the change of emission intensity reflects the population change. Figure 2b shows the dependences of normalized integrated emission intensities for all observed transitions on the Er3+ concentration. From Fig. 2b it can be seen that the emission intensity of the metastable levels 2H11/2/4S3/2 reaches its maximum at around 5 mol% of Er3+. Therefore, the 5 mol% of Er3+ concentration is suggested for designing the Er3+/Yb3+ co-doped NaY(WO4)2 quantum cutting phosphors. In addition, the 4F9/2 as a metastable level may also get involved in the quantum cutting process, but its population under 378 nm excitation is much smaller than that of the levels 2H11/2/4S3/2. Therefore, the contribution of level 4F9/2 to the quantum cutting would be less and can be ignored. Moreover, from Fig. 2b the 2H9/2 level shows very gentle fluorescence concentration quenching, but its influence on the quantum cutting process is limited since its population is very less in comparison with levels 2H11/2/4S3/2.
Quantum cutting mechanisms
In this section, we will explore the quantum cutting mechanisms in Er3+/Yb3+ co-doped NaY(WO4)2 phosphors. To this end, the emission spectra of Er3+ and Yb3+ co-doped NaY(WO4)2 were measured under 378 nm excitation and are shown in Fig. 3a, b whose wavelength ranging from 500 to 750 nm and from 800 to 1700 nm, respectively. The insert of Fig. 3a displays the enlarged emission spectra in the wavelength range from 600 to 750 nm. It should also be mentioned that there are no emissions observed in the wavelength shorter than 500 nm. This implies that the 2H11/2/4S3/2 levels are populated mainly via cascade nonradiative relaxations from 4G11/2. This also means that the quantum cutting for the levels lying between 4G11/2 and 2H11/2 will not happen since these levels were not effectively populated.
a Visible emission spectra, b near infrared emission spectra, c dependence of normalized visible emission intensity on Yb3+ concentration, and d dependence of infrared emission intensities of Yb3+ and Er3+ on Yb3+ concentration for NaY(WO4)2: 5 mol% Er3+ / x mol% Yb3+ phosphors under 378 nm excitation, e energy level diagram and quantum cutting routes of Er3+/Yb3+ doped NaY(WO4)2 phosphors
From Fig. 3a it can be seen that the samples with varied Yb3+ concentrations exhibit four emissions as observed in the Er3+ single-doped samples. Figure 3c, d show the dependences of the three visible emission and two NIR emission intensities on the Yb3+ concentration, respectively. It is found that though the Er3+ concentration is fixed, the emission intensity of 2H11/2/4S3/2→4I15/2 decreases with increasing Yb3+ concentration. This fact means that the Yb3+ plays a role of depopulating 2H11/2/4S3/2 levels, that is to say an energy transfer from Er3+ to Yb3+ occurs. Based on the energy matching rule, the energy transfer path can be confirmed as ET1: 4S3/2(Er3+)+2F7/2(Yb3+) →4I11/2(Er3+) + 2F5/2(Yb3+). The large energy mismatch between the energy difference from 4S3/2 to 4I11/2 and the energy difference from 2F7/2 to 2F5/2 means the phonon-assisted energy transfer is involved, and the ET1 happens by annihilating lattice phonons to maintain the energy conservation. After this energy transfer one Er3+ gets into 4I11/2 and one Yb3+ gets into 2F5/2. Analogically, from the dependence of red emission intensity on the Yb3+ concentration, an energy transfer 4F9/2(Er3+)+ 2F7/2(Yb3+)→4I13/2(Er3+)+2F5/2(Yb3+) can be deduced, and this energy transfer process is not drawn in Fig. 3e since its contribution to the quantum cutting is very less owing to the ignorable population of 4F9/2 of Er3+ under 378 nm excitation. From Fig. 3b it can be seen that the emission intensity of 2H9/2 level is almost not changed with Yb3+ concentration, thus indicating that there is no any energy transfer originating from 2H9/2 level. It should also be stated that the impact of 2H9/2 level on the quantum cutting will also be ignored since the population of this level is less too.
By inspecting the energy level scheme of Er3+ and Yb3+ in Fig. 3e, it can be deduced that the infrared emission intensity of Yb3+ would increase with the increase of Yb3+ concentration as a result of the energy transfer ET1, at the same time, the infrared emission intensity of Er3+ (corresponding to 4I13/2→4I15/2 transition) should synchronously increase due to the increase of 4I13/2 level population via a nonradiative relaxation from 4I11/2 level. From the dependences of integrated emission intensities of 2F5/2→2F7/2 (Yb3+) and 4I13/2→4I15/2 (Er3+) transitions on the Yb3+ concentration in Fig. 3d, it is found that Yb3+ emission intensity does increase with increasing Yb3+ concentration as we predicted, but the intensity of 4I13/2→4I15/2 (Er3+) transition decreases with increasing Yb3+ concentration which is against our prediction. The decrease of 4I13/2→4I15/2 (Er3+) transition implies the decrease of 4I13/2 population with increasing Yb3+ concentration. Owing to large energy mismatch, the energy transfer 4I13/2 (Er3+) + 2F7/2 (Yb3+) → 4I15/2 (Er3+) + 2F5/2 (Yb3+) cannot happen. Therefore, the energy transfer 4I11/2 (Er3+) + 2F7/2 (Yb3+) → 4I15/2 (Er3+) + 2F5/2 (Yb3+) (marked as ET2 in Fig. 3e) would occur indubitably. It should be pointed out that if ET2 does not exist, the intensity of 4I13/2→4I15/2 (Er3+) transition will increase following the same trend as the Yb3+ emission intensity does. This is because that the 4I13/2 is populated via both radiative transition 4I11/2 → 4I13/2 and nonradiative relaxation from 4I11/2, and the 4I11/2 is populated via the energy transfer ET1 which evokes the increase of Yb3+ emission intensity with increasing Yb3+ concentration. It should also be noticed that if the population of 4I11/2 gained via ET1 process is totally contributed to Yb3+ via ET2 process, then with increasing Yb3+ concentration the intensity of 4I13/2→4I15/2 transition will keep a constant intensity as in the 5 mol% Er3+ single-doped sample. If the population of 4I11/2 gained via ET1 process is partially contributed to Yb3+ via ET2 process and partially contributed to 4I13/2 via radiative transition and nonradiative relaxation from 4I11/2, then the infrared emission of 4I13/2→4I15/2 transition will also show an increasing trend when Yb3+ concentration increases. The decrease of infrared emission intensity of 4I13/2→4I15/2 transition with increasing Yb3+ concentration indicates great enhancement of the energy transfer rate of ET2 process with increasing Yb3+ concentration.
Based on the above analyses, the specific photon-splitting routes are illustrated in Fig. 3e. In the first step, the Er3+ in the ground state 4I15/2 is promoted to the excited state 4G11/2 by absorbing a 378 nm photon, and then relaxes rapidly to 2H11/2/4S3/2 states. The Er3+ in 2H11/2/4S3/2 states could be de-excited via three possible routes, namely radiative transition 2H11/2/4S3/2→4I15/2, nonradiative relaxation from 4S3/2 to 4F9/2, and energy transfer ET1 4S3/2(Er3+)+2F7/2(Yb3+)→4I11/2 (Er3+) + 2F5/2(Yb3+). The radiative transitions generate the green emissions as shown in Fig. 1a, and the nonradiative relaxation results in the population of 4F9/2 and further induces the very weak red emission as shown in the insert of Fig. 1a. The ET1 process can eventuate simultaneously two effects that infrared emission of Yb3+ and population of 4I11/2 of Er3+. The Er3+ in 4I11/2 can be de-excited via radiative transitions 4I11/2→4I15/2 and 4I11/2→4I13/2, nonradiative relaxation to 4I13/2, and energy transfer ET2. The radiative transition 4I11/2→4I15/2 can generate 980 nm photons. The radiative transition 4I11/2→4I13/2 and nonradiative relaxation from 4I11/2 to 4I13/2 will result in 1550 nm emission. The ET2 process generate infrared emission of Yb3+.
Quantum cutting efficiency
This section will focus on quantitatively estimating the quantum cutting efficiency. It should be mentioned that the energy transfer efficiency from Er3+ to Yb3+ cannot be calculated in the way as done for Tb3+/Yb3+ co-doped system since the energy transfer mechanisms are different. To calculate the quantum cutting efficiencies, the nonradiative transitions from 4I13/2 to 4I15/2 and from 2F5/2 to 2F7/2 are ignored, but the nonradiative transitions in the possible cases are considered. The radiative transitions are quantitatively evaluated in the framework of Judd-Ofelt calculation.
Energy transfer efficiency for ET1 from Er3+ to Yb3+
The energy transfer efficiency of ET1 can be written as
where \({I}^{{Er}}\) and \({I}^{{Er}+{Yb}}\) are the luminescence intensities of 4S3/2→4I15/2 for 5 mol% Er3+ single-doped and 5 mol% Er3+/ x mol% Yb3+ co-doped samples. By taking the integrated luminescence intensity data in Fig. 3b into Eq. (1) the energy transfer efficiencies were calculated, and the dependence of energy transfer efficiency on the Yb3+ concentration is shown in Fig. 4a. It can be seen that the energy transfer efficiency dramatically increases with increasing Yb3+ concentration, and the energy transfer efficiency can reach 70% when Yb3+ concentration is 50 mol%.
a Dependence of energy transfer efficiency of ET1 on Yb3+ concentration, b Dependence of nonradiative relaxation rate on energy gap: experimental data (solid squares) and fitting curve (dashed), c Solid squares show the relationship between the energy transfer efficiency for ET2 and Yb3+ concentration; Solid circles show the relationship between the quantum cutting efficiency and Yb3+ concentration
Radiative transition rates of Er3+ in NaY(WO4)2
From the quantum cutting mechanism shown in Fig. 3e, it can be known that to quantitatively calculate the quantum cutting efficiency, the radiative transition rates between 4 f levels of Er3+ should be determined. To this end, the Judd-Ofelt calculation for Er3+ in NaY(WO4)2 host should be carried out in advance. In this work, the Judd-Ofelt parameters were obtained via an approach proposed by Zhan Y et al. 46,47 using the diffuse reflection spectrum. The specific calculation procedure is presented in supporting information file as the Item <Judd-Ofelt calculation>48,49, and the calculation results are listed in Table 1.
Nonradiative transition rates of Er3+ in NaY(WO4)2
From Fig. 3e it can be found that the nonradiative transitions are involved in the quantum cutting process, and some of them, for example, the nonradiative transition of 4I11/2 level, play an important role. Moreover, the cascade nonradiative transitions (NR1, NR2, and NR3) are a pathway for populating 4I11/2 level. Therefore, to determine the quantum cutting efficiency, the nonradiative transition rates for some levels should be determined in advance.
At a certain temperature, the nonradiative transition rate \({A}_{{nr}}\) of an energy level can be expressed by as follows,
where C is temperature-dependent parameter, and at a not-too-high temperature the parameter C can be considered as a constant independent from \(\triangle E\); \(\alpha\) is a constant in a certain system; \(\triangle E\) is the energy gap between the studied energy level and its most adjacent lower energy level. To confirm these parameters C and \(\alpha\), the fluorescence decays of 4F5/2, 4F9/2, and 4I9/2 levels for 1 mol% Er3+ doped NaY(WO4)2 phosphor were measured and are shown in Fig. S4. It can be seen that all decays display straight lines in the semi-logarithmic coordinate system, thus implying all decays follow the mono-exponential function. Therefore, mono-exponential function was fit to these decays, and the fluorescence lifetimes were confirmed and are marked in the figure. In the sample single-doped with 1 mol% Er3+, the energy transfers between Er3+ can be ignored since the distance between Er3+ is large enough. Therefore, the nonradiative relaxation rate for a level can be expressed as
where \({\tau }_{{ex}}\) is fluorescence lifetime, \({A}_{r}\) is total radiative transition rate as listed in Table 1. By taking the lifetimes obtained from Fig. S4 into Eq. (3), the nonradiative relaxation rates for 4F5/2, 4F9/2, and 4I9/2 levels are confirmed, and the energy gaps from 4F5/2, 4F9/2, and 4I9/2 levels to their own most adjacent lower levels can be obtained from Table 1. The dependence of the nonradiative relaxation rate on the energy gap is shown in Fig. 4b as the solid squares. The data in Fig. 4b were fit to Eq. (2), and the values of the free parameters \(C\) and \(\alpha\) were confirmed from the fitting process to be 1.9×106 s-1 and 6.8×10-4 cm. Thereby the nonradiative relaxation rates for all level can be derived by taking energy gap value between the studied level and its most adjacent level into Eq. (2). The nonradiative relaxation rates for interested levels of Er3+ in NaY(WO4)2 were calculated and are listed in Table 1 as the second column from right hand. It should be stated that the 2H11/2 and 4S3/2 were not involved in the determination of the parameters \({\rm{C}}\) and \({\rm{\alpha }}\) since they are in a thermal equilibrium, and 4I13/2 was not involved either since the distance between 4I13/2 and 4I15/2 is too large.
Energy transfer efficiency for ET2 from Er3+ to Yb3+
The energy transfer rate for ET2 could be calculated based on the Fӧrster-Dexter energy transfer theory, the energy transfer rate \({A}_{{et}}^{D\to A}\) between donors and acceptors can be expressed as follows49,50,
where \({A}_{{et}}^{D\to A}\) is the energy transfer rate for ET2 process from donor Er3+ to acceptor Yb3+, \(h\) is Planck constant, \(c\) is the velocity of light in vacuum, \({Q}_{A}\) and \({Q}_{D}\) are the integrated cross-sections for acceptor absorption (Yb3+: 2F7/2→2F5/2) and donor emission (Er3+: 4I11/2→4I15/2), \({f}_{A}\left(E\right)\) and \({f}_{D}(E)\) are the normalized spectral line shape functions of acceptor absorption and donor emission, respectively. \(n\) is the refractive index of the host NaY(WO4)2. \(E\) is the photon energy, namely hv, and \(R\) is average distance between Er3+ and Yb3+ ions in NaY(WO4)2 and can be calculated based on the following formula51,
where \({N}_{A}\) and \({N}_{D}\) are numbers of Yb3+ and Er3+ ions in a unit volume, respectively.
The emission cross-section spectrum \({\sigma }_{{em}}^{{Er}}({\rm{\lambda }})\) for 4I11/2→4I15/2 transition was calculated based on the Füchtbauer-Ladenburg and is plotted in Fig. S5 as dashed curve. The absorption cross-section \({\sigma }_{{ab}}^{{Yb}}\left({\rm{\lambda }}\right)\) for 2F7/2→2F5/2 transition of Yb3+ was calculated by below formula,
where \({\alpha }_{{Yb}}^{{\prime} }\left({\rm{\lambda }}\right)\) is the spectral intensity for 2F7/2→2F5/2 transition of Yb3+ at wavelength \({\rm{\lambda }}\), \({{A}}_{4I13\to 4I15/2}^{{MD}}\) is magnetic dipole allowed transition rate for 4I13/2→4I15/2 transition and can be found in Table 1, \({A}_{4I13\to 4I15/2}^{{\prime} {ED}}\) is relative radiative electric dipole allowed transition rate for 4I13/2→4I15/2 transition and can be calculated directly from Eq. (S6). The reason for the insert of coefficient \(\frac{1}{2}\) in Eq. (6) is that the Yb3+ concentration is two times the Er3+ concentration. By taking the corresponding data in Fig. S2 into Eq. (6), the emission cross-section \({\sigma }_{{ab}}^{{Yb}}\left({\rm{\lambda }}\right)\) was confirmed and plotted in Fig. S5 as solid curve. It should be mentioned that in Fig. S2 the absorption peak centered at 980 nm contains both contributions of the transition 4I15/2→4I11/2 of Er3+ and the transition 2F7/2→2F5/2 of Yb3+, and in Eq. (6) the \({\alpha }_{{Yb}}^{{\prime} }\left({\rm{\lambda }}\right)\) was derived by subtracting the spectral intensity of 5 mol% Er3+ single-doped sample from spectral intensity of 5 mol% Er3+/10 mol% Yb3+ co-doped sample. According to the above calculation procedure, the energy transfer rates for all the samples with different Yb3+ concentrations were calculated, and then the energy transfer efficiencies were calculated via following formula,
where \({A}_{r}^{4I11/2}\) is the sum of the radiative transition rates of Er3+ from 4I11/2 to 4I13/2 and 4I15/2 (the values are listed in Table 1), \({A}_{{nr}}^{4I11/2}\) is the nonradiative transition rate of 4I11/2 of Er3+ (the value is also listed in Table 1). The dependence of energy transfer efficiency on the Yb3+ is shown in Fig. 4c as solid squares, and the dashed curve shows the changing trend.
Quantum cutting efficiency
In the above sub-Sections, all the depopulation rates including radiative transition rates, nonradiative transition rates, and energy transfer rates are derived, therefore the quantum cutting efficiency \({{\rm{\eta }}}_{QC}\) of Er3+/Yb3+ co-doped NaY(WO4)2 phosphors can be calculated via following formula,
where \({{\rm{\eta }}}_{{Yb}}\) is the luminescence quantum efficiency of Yb3+ in NaY(WO4)2, \({{\rm{\eta }}}_{{Multi}}\) is the efficiency for populating 4I11/2 via cascade nonradiative relaxations of 4S3/2 (see NR1, NR2 and NR3 in Fig. 3e) and multi-step radiative transitions from 2H112, 4S3/2, 4F9/2, and 4I9/2 to 4I11/2. The \({{\rm{\eta }}}_{{Multi}}\) is dependent on the radiative and nonradiative transitions of Er3+ in NaY(WO4)2 but independent from Yb3+ concentration. The nonradiative relaxation rate of 2F5/2 of Yb3+ can be ignored owing to the large enough energy gap between 2F7/2 and 2F5/2. The energy transfer rate from Yb3+ to any luminescence centers or quenching centers can also affect the luminescence quantum efficiency of Yb3+. To evaluate the influence of energy transfer from Yb3+ to other centers, the fluorescence decays of Yb3+ were measured under 451 nm excitation and are shown in Fig. S6. It is found that the fluorescence decay does not change with Yb3+ concentration, therefore \({{\rm{\eta }}}_{{Yb}}\) is taken as 1 in calculating the quantum cutting efficiency. In addition, \({{\rm{\eta }}}_{{Multi}}\) value can be calculated by using the radiative transition rates and nonradiative relaxation rates in Table 1, and the value of \({{\rm{\eta }}}_{{Multi}}\) is found to be 22.5%. In the calculation it is found that the contribution of the multi-step radiative transitions is very small since the radiative transition branching ratios from 2H11/2, 4S3/2, 4F9/2 and 4I9/2 to 4I11/2 are very small. Based on the above analyses, the quantum cutting efficiencies for all the samples with different Yb3+ concentrations were calculated, and the dependence of the quantum cutting efficiency on Yb3+ concentration is shown in Fig. 4c as solid circles. From Fig. 4c it can be seen that around 173% of quantum cutting efficiency can be realized in Er3+/Yb3+ co-doped NaY(WO4)2 phosphor when the Er3+ and Yb3+ concentrations are 5 mol% and 50 mol%, thus indicating Er3+/Yb3+ co-doped NaY(WO4)2 phosphor is an excellent quantum cutting material. It should be mentioned that the quantum cutting efficiency calculated in this work is different from the experimentally measured efficiency. The calculated efficiency is internal quantum efficiency that means it does not conclude the transmission efficiency of excitation light and emission light in the host material. Therefore, the calculated quantum cutting efficiency is usually larger than or close to the experimentally measured efficiency.
980 nm up-conversion emission under 1550 nm excitation
It is well known that the Er3+ doped materials can convert the 1550 nm photons into solar-cell-absorbable photons including visible and near-infrared photons via the so-called frequency upconversion processes. In the previous Section it was discovered that the energy transfer ET2 from Er3+ to Yb3+ is very efficient in the co-doped samples. Therefore, it is expected that the energy transfer ET2 could also take effect and result in efficient Yb3+ emission in the upconversion process when the co-doped samples are excited at 1550 nm.
Concentration effects of upconversion luminescence
In this section the concentration dependence of upconversion emission intensity will be examined. Fig. S7 shows the upconversion emission spectra in the wavelength range from 400 nm to 1100 nm for the samples with different Er3+ concentration, and the insert shows the enlarged upconversion emission spectra in the wavelength range from 400 nm to 900 nm. It can be seen that under 1550 nm excitation the Er3+ single-doped NaY(WO4)2 phosphors mainly emit 999 nm infrared light corresponding 4I11/2→4I15/2 transition of Er3+, and the other upconversion emissions corresponding 2H11/2/4S3/2→4I15/2, 4F9/2→4I15/2 and 4I9/2→4I15/2 are extremely weak. The dispersed solid squares in Fig. 5a show the dependence of 999 nm upconversion intensity on the Er3+ doping concentration. It is found that the upconversion emission intensity monotonically increases but its growing rate decreases with increasing Er3+ concentration. As a compromise between the quantum cutting and the upconversion, the concentration selection of 5 mol% Er3+ is also reasonable for further introducing Yb3+. Figure S8 shows the upconversion emission spectra for the samples doped with fixed 5 mol% Er3+ and varied Yb3+ concentrations (0, 0.5, 1, 2, 5, 10, 20, and 50 mol%). It can be seen that nearly pure infrared emission of Yb3+ at 999 nm accompanying with very weak visible emissions was observed and the infrared emission intensity increases with increasing Yb3+ concentration. The insert of Fig. S8 shows the enlarged spectra ranging from 400 nm to 900 nm. Figure 5b shows the dependence of the infrared emission integrated intensity on the Yb3+ concentration. From Fig. 5b it can be seen that the Yb3+ introduction greatly increases the infrared emission, and the infrared upconversion intensity of 5 mol% Er3+/50 mol% Yb3+ co-doped phosphor is 20 times higher than the intensity of 5 mol% Er3+ single-doped phosphor. The concentration quenching for the upconversion process is different from the down-shifting process since the concentration quenching depends on the excitation wavelength. Different excitation wavelengths result in different populating and depopulating pathways.
Excitation power dependences of upconversion luminescence
From Figs. S7 and S8 it can be found that the upconversion spectra are almost not changed except for the spectral intensities, thus indicating the upconversion mechanisms are not changed with doping concentration. To discover the upconversion mechanism, the upconversion emission spectra for 5 mol% Er3+ single-doped NaY(WO4)2 phosphor excited at varied excitation power were measured. In this work, the excitation power of the 1550 nm laser was controlled by changing working current of the laser. Fig. S9a shows the upconversion emission spectra measured at different working currents. The insert of Fig. S9a displays the enlarged upconversion spectra ranging from 500 nm to 600 nm for 2H11/2/4S3/2→4I15/2 transitions. The relation between the fluorescence intensity ratio of 2H11/2→4I15/2 to 4S3/2→4I15/2 and the working current is depicted in Fig. S9b. It can be seen that the fluorescence intensity ratio does not change with increasing working current, thus implying the sample temperature was kept as a constant. This constant temperature means that there is no fluorescence temperature quenching caused by laser irradiation in the spectral measurements. Therefore, the excitation-power-dependent spectra are reliable for deducing the upconversion mechanism. The dependence of the integrated upconversion intensity for 4I11/2→4I15/2 emission on the working current was derived from the spectra in Fig. S9a and is shown in Fig. S9c. The data in Fig. S9 was fit to the following function52.
where \(I\left(i\right)\) is the integrated upconversion intensity at working current \(i\), a is a constant, \(n\) is the excitation photon number needed for emitting one 999 nm photon, \({i}_{0}\) is the threshold current of the 1550 nm laser. From the fitting process, the n was confirmed to be 2.13 which is close the theoretical value for the two-photon process, thus implying the 999 nm upconversion emission is a two-photon process.
In an analogical way, the upconversion spectra for the samples doped with 5 mol% Er3+ and 10 mol% Yb3+ were also measured under 1550 nm excitation and are shown in Fig. S10a. The unchanged fluorescence ratios at different currents were also observed as seen in Fig. S10b. The similar fitting operation was also carried out for the data in Fig. S10c, and a two-photon process was also confirmed to be dominant for Yb3+ upconversion emission.
Upconversion mechanism
From the upconversion spectra and the excitation-power-dependent upconversion intensity, the possible upconversion mechanisms for Er3+ and Er3+/Yb3+ doped NaY(WO4)2 phosphors are portrayed in Fig. 5c. In Er3+ single-doped NaY(WO4)2 phosphors under 1550 nm excitation the 4I13/2 can be populated via ground state absorption. The 4I9/2 is mainly populated via an energy transfer et1 since the excited state absorption 4I13/2→4I9/2 is rather weak owing to the large energy mismatch and small transition rate of 4I13/2→4I9/2 (see Table 1). 4I9/2 is mainly depopulated via a nonradiative relaxation to 4I11/2, therefore the intense 4I11/2→4I15/2 upconversion emission could be observed in the Er3+ single-doped samples. The 4F9/2 is mainly populated via an energy transfer et2, but the energy transfer rate is relatively weak owing to the large energy mismatch though the 4I11/2 is heavily populated via a nonradiative relaxation from 4I9/2 to 4I11/2. Therefore, the red upconversion emission is weak. The 4S3/2 is populated via an energy transfer et3, but the population of 4I9/2 is less owing to the strong nonradiative transition. Therefore, the green emissions of 4S3/2/2H11/2→4I15/2 are also weak.
When Yb3+ is introduced, the population pathways of Er3+ under 1550 nm excitation will be the same as in Er3+ single-doped NaY(WO4)2 phosphors. It should be mentioned that we have not observed any emissions in the Yb3+ single-doped NaY(WO4)2 phosphors (even with high concentration). However, Yb3+ will accept the energy transferred from Er3+ since the energy transfer efficiency for et4 in Fig. 5c is very large as discovered in the above Section where it was stated as ET2. Therefore, the 4I11/2→4I15/2 emission decreases but 2F5/2→2F7/2 of Yb3+ increases. These upconversion mechanisms tell us that both Er3+ and Er3+/Yb3+ doped NaY(WO4)2 phosphors exhibit strong near infrared emissions from 4I11/2→4I15/2 of Er3+ and 2F5/2→2F7/2 of Yb3+ that indicates the studied phosphors are good light conversion candidate for silicon-based solar cell applications. In this work, the quantum cutting and upconversion processes were individually studied, but in practical application these two processes co-happen at the same time. The quantum cutting and upconversion processes are dependent on the energy level distribution of the luminescence centers. The quantum cutting and upconversion processes can exist as long as the energy level structure does not change. However, the efficiencies of the quantum cutting and upconversion processes are different in these two cases: co-happen and solo-exist because the population processes in the co-happened case are different from the studied cases.
Optical temperature sensing
When the NaY(WO4)2:Er3+/Yb3+ is introduced into the silicon-based photovoltaic system, the spectral conversions including quantum cutting and upconversion could improve the photoelectric conversion efficiency, and simultaneously the system temperature could also be read out via a fluorescence intensity ratio technique as we stated in the Introduction section. Therefore, in this section, the temperature sensing performance of NaY(WO4)2:Er3+/Yb3+ will be assessed.
Temperature sensing properties based on fluorescence intensity ratio
For a certain Er3+ doped system, the temperature sensing properties are usually independent from doping concentration unless the optical transition property of Er3+ changes greatly with doping concentration53. To examine the temperature sensing, the 5 mol% Er3+ and 10 mol% Yb3+ co-doped NaY(WO4)2 phosphor was selected for study and its green emission spectra were measured at different temperatures from 300 K to 720 K under 378 nm excitation and are shown in Fig. S11. The integrated emission intensities of 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions were calculated. The solid squares, solid circles, and triangles in Fig. 6a show the temperature-dependent fluorescence intensities for 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions and their sum. It can be seen that 4S3/2→4I15/2 emission intensity monotonically decreases with increasing sample temperature, and this decrease can be attributed to its two enhanced depopulation rates, namely nonradiative relaxation rate to 4F9/2 and thermal population rate to 2H11/2. The 2H11/2→4I15/2 emission intensity increases first and then decreases with increasing temperature that is caused by two competing population and depopulation processes. The population process is the thermal excitation of 4S3/2 which leads to enhancing emission intensity, and the depopulation process is the decrease of the sum population of 2H11/2 and 4S3/2 due to nonradiative relaxation of 4S3/2 which leads to depressing the emission intensity. From Fig. 6a it can be found that the studied phosphors exhibit excellent temperature quenching that the sum of two green emission intensities shows a decrease of only 25.8% when the temperature increases from 300 K to 720 K. This excellent temperature quenching performance of the studied phosphors benefits the application in solar cells since the solar cells are often work at high temperatures.
Fluorescence thermal properties of 5 mol% Er3+ and 10 mol% Yb3+ co-doped NaY(WO4)2 phosphor a temperature-dependent up-conversion emission intensities: ■ for 2H11/2→4I15/2, ● for 4S3/2→4I15/2, and ▲ for the sum of both; b fluorescence intensity ratio of 2H11/2→4I15/2, to 4S3/2→4I15/2: Dispersed solid squares are the experimental data, solid curve is the fitting curve; c Functions of relative and absolute sensitivities versus temperature
The solid squares in Fig. 6b show the fluorescence intensity ratio of 2H11/2→4I15/2 transition to 4S3/2→4I15/2 transition. The data in Fig. 6b was fit to the following formula54,55
where \({I}_{H}\) and \({I}_{S}\) are the green emission intensities of 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions, \(\triangle E\) is the energy distance between 2H11/2 and 4S3/2. The parameter values of \(C\) and \(\triangle E/k\) were determined to be 17.13 and 886.46 K via fitting Eq. (10) to the data in Fig. 6b. Once these parameters are confirmed, the NaY(WO4)2:Er3+/Yb3+ can be used as a temperature probe. To evaluate the temperature sensing performance the absolute (\({S}_{A}\)) and relative (\({S}_{R}\)) sensitivities are calculated via the following functions54,55
By taking the parameter values of \(C\) and \(\triangle E/k\) into Eqs. (11) and (12), the absolute and relative sensitivities are derived and are plotted in Fig. 6c. The maximum value of \({S}_{A}^{\max }\) and its corresponding temperature value \({T}_{\max }\) can be determined via a simple mathematical treatment to be \({S}_{A}^{\max }=4\frac{{Ck}}{{e}^{2}\triangle E}\) and \({T}_{\max }=\frac{\triangle E}{2k}\)54 which are marked in Fig. 6c. Usually, the solar cells operate at the temperature lower than 573 K (300 °C) since at the temperature higher than 573 K the solar cells will be damaged. From Fig. 6c it can be seen that in the temperature range from room temperature 300 K (27 °C) to 573 K (300 °C) the studied NaY(WO4)2:Er3+/Yb3+ works in the status with the best absolute and relative sensitivities in its possible working temperature range.
Temperature quenching mechanism of green emissions of Er3+ in NaY(WO4)2 phosphors
In Fig. 6a the temperature quenching of green emissions of Er3+ in NaY(WO4)2 was observed, but the temperature quenching mechanism is not clear so far. If the nonradiative relaxation of 4S3/2 is the main depopulation route, then the fluorescence intensity \({I}_{s}\) of 4S3/2 can be theoretically expressed as follows,
where \({A}_{r}\) is radiative transition rate of 4S3/2→4I15/2 transition, \(a\) is temperature-independent constant, \({A}_{{all}}\) is the sum of all depopulation rates of 4S3/2 except for the nonradiative relaxation rate, and \({W}_{{nr}}(0){\left[1-\exp \left(-{hv}/{kT}\right)\right]}^{-p}\) is the nonradiative relaxation rate of 4S3/2. Here, \({W}_{{nr}}(0)\) is the nonradiative relaxation rate at a temperature infinitely close to 0 K. \(v\) is the effective phonon frequency, \(p\) stands for the generated phonon number in the nonradiative relaxation process. By combining Eqs. (10) and (13), it can be derived that
where \(b\) is a constant independent from temperature. Eqs. (14) and (15) were fit to the data for 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions in Fig. 6a. The solid curves in Fig. 6a are the fitting curves, and it can be seen that Eqs. Eqs. (14) and (15) fit in well with the experimental data. Moreover, the function of \({I}_{H}+{I}_{S}\) was fit to the data for fluorescence sum of 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions, and the solid curve shows the fitting curve. In this fitting process the parameters \(C\) and \(\triangle E/k\) were fixed as derived from Eq. (10), and the parameters \({hv}/k\) and \(p\) were taken as free parameters. From two fitting processes for 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions, the parameters \({hv}/k\) and \(p\) were determined to be 1245.0 K and 3.6. Therefore, the maximum phonon energy \({{dE}}_{\omega }\) of the NaY(WO4)2 can be estimated to be 889.3 cm-1 which is very close the experimental value of 900 cm-1. From Table 1 it is known that the energy difference \({dE}\) between 4S3/2 and 4F9/2 is around 3091.83 cm-1, therefore the generated phonon number in the multiphonon nonradiative relaxation of 4S3/2 is \({dE}/{{dE}}_{\omega }\approx 3.4\) which is close to the \(p\) value of 3.6, thus implying fluorescence quenching of 2H11/2/4S3/2→4I15/2 is caused by the nonradiative relaxation of 4S3/2.
Conclusions
In summary, tri-functionalization including quantum cutting, infrared upconversion, and temperature detection was realized in Er3+/Yb3+ co-doped NaY (WO4)2 phosphors. Based on the optical spectroscopic analyses, the quantum cutting mechanism was discovered, and the photon splitting process includes two-step energy transfer processes, namely, 4S3/2 + 2F7/2 →4I11/2 + 2F5/2 and 4I11/2 + 2F7/2→ 4I15/2 + 2F5/2. Furthermore, the radiative transition rates for all interested transitions of Er3+ were calculated in the framework of Judd-Ofelt theory, and the nonradiative transition rates for all concerned energy levels were also derived according to the energy gap law. Starting from the radiative transition rates, nonradiative transition rates, and experimental fluorescence lifetimes of correlated energy levels, the quantum cutting efficiencies were calculated, and the concentration-dependent maximum quantum cutting rate was found to be as high as 173%. Under 1550 nm excitation, the near infrared emissions of 4I11/2→4I15/2 and 2F5/2→2F7/2 from Er3+ single-doped and Er3+/Yb3+ co-doped NaY(WO4)2 phosphors were observed, respectively. It was found that all samples with various doping concentrations showed nearly pure-color near infrared emissions, and the possible upconversion mechanisms were proposed. The studied materials displayed excellent thermal quenching of 4S3/2→4I15/2 emission that is beneficial to the quantum cutting. The thermal quenching mechanism was assigned to the nonradiative transition of 4S3/2. Moreover, the temperature sensing study proved that the Er3+/Yb3+ co-doped NaY(WO4)2 phosphors presented excellent temperature sensing performance. All these results imply that Er3+/Yb3+ co-doped NaY(WO4)2 phosphors are excellent light-converted and temperature sensing material having potential application in silicon-based solar cells to improve photovoltaic performance.
References
Höök, M. & Tang, X. Depletion of fossil fuels and anthropogenic climate change-a review. Energy Policy 52, 797–809 (2013).
Tamilselvan, P., Nallusamy, N. & Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 79, 1134–1159 (2017).
Lee, J. et al. Hybrid renewable energy systems involving thermochemical conversion process for waste-to-energy strategy. Chem. Eng. J. 452, 132918 (2023).
Bie, P. J. et al. A review and evaluation of nonroad diesel mobile machinery emission control in China. J. Environ. Sci. 123, 30–40 (2023).
Raheem, I. et al. Rapid growth of MXene-based membranes for sustainable environmental pollution remediation. Chemosphere 311, 137056 (2023).
González-Torres, M. et al. A review on buildings energy information: trends, end-uses, fuels and drivers. Energy Rep. 8, 626–637 (2022).
Abdelfattah, A. et al. Microalgae-based wastewater treatment: mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 13, 100205 (2023).
Lin, Y. H. et al. Alleviating the self-discharge and enhancing the polysulphides conversion kinetics with LaCO3OH nanocrystals decorated hierarchical porous carbon. Chem. Eng. J. 452, 139091 (2023).
Kumar, A. et al. A review on S-scheme and dual S-scheme heterojunctions for photocatalytic hydrogen evolution, water detoxification and CO2 reduction. Fuel 333, 126267 (2023).
Salamah, T. et al. Effect of dust and methods of cleaning on the performance of solar PV module for different climate regions: comprehensive review. Sci. Total Environ. 827, 154050 (2022).
Wu, N. et al. Efficient furan-bridged dibenzofulvene-triphenylamine hole transporting materials for perovskite solar cells. Mater. Adv. 4, 515–522 (2023).
Zhao, J. L. et al. Photochromic crystalline hybrid materials with switchable properties: recent advances and potential applications. Coord. Chem. Rev. 475, 214918 (2023).
Ma, Z. X. et al. Efficient decontamination of organic pollutants from wastewater by covalent organic framework-based materials. Sci. Total Environ. 901, 166453 (2023).
Liao, G. F. et al. Z-scheme systems: from fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 983, 1–41 (2022).
Mehta, N. et al. Down-conversion of a single photon as a probe of many-body localization. Nature 613, 650–655 (2023).
Arduini, F. et al. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio)sensor design. Biosens. Bioelectron. 156, 112033 (2020).
Tian, N. et al. Layered bismuth-based photocatalysts. Coord. Chem. Rev. 463, 214515 (2022).
Petrov, V. Frequency down-conversion of solid-state laser sources to the mid-infrared spectral range using non-oxide nonlinear crystals. Prog. Quant. Electron. 42, 1–106 (2015).
Hola, K. et al. Carbon dots-Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 9, 590–603 (2014).
Pulli, E., Rozzi, E. & Bella, F. Transparent photovoltaic technologies: current trends towards upscaling. Energy Convers. Manag. 219, 112982 (2020).
Jia, Y. T., Alva, G. & Fang, G. Y. Development and applications of photovoltaic–thermal systems: a review. Renew. Sustain. Energy Rev. 102, 249–265 (2019).
Alami, A. H. et al. Management of potential challenges of PV technology proliferation. Sustain. Energy Technol. Assess. 51, 101942 (2022).
Mojiri, A. et al. Spectral beam splitting for efficient conversion of solar energy-a review. Renew. Sustain. Energy Rev. 28, 654–663 (2013).
Raijmakers, L. H. J. et al. A review on various temperature-indication methods for Li-ion batteries. Appl. Energy 240, 918–945 (2019).
Abram, C., Fond, B. & Beyrau, F. Temperature measurement techniques for gas and liquid flows using thermographic phosphor tracer particles. Prog. Energy Combust. Sci. 64, 93–156 (2018).
Taylor, N. A. S., Tipton, M. J. & Kenny, G. P. Considerations for the measurement of core, skin and mean body temperatures. J. Therm. Biol. 46, 72–101 (2014).
Roy, A. et al. The impact of pure and mixed self-activated YXO4 phosphor materials (X=V, Nb and Ta) on downshifting and quantum cutting emission behaviours of Ln3+ doped (Ln3+=Ho3+ and Yb3+) ions. Ceram. Int. 49, 17383–17395 (2023).
Mishra, N. K. et al. Probing multimodal light emission from Tb3+/Yb3+-doped garnet nanophosphors for lighting applications. Phys. Chem. Chem. Phys. 25, 11756–11770 (2023).
Li, D. C. et al. Quantum cutting in KGd(CO3)2: Tb3+ green phosphor. Nanomaterials 13, 351 (2023).
Balaji, S. et al. Insights into Er3+↔Yb3+ energy transfer dynamics upon infrared ~1550 nm excitation in a low phonon fluoro-tellurite glass system. J. Lumin. 187, 441–448 (2017).
Wegh, R. T. et al. Visible quantum cutting in LiGdF4: Eu3+ through downconversion. Science 283, 663–666 (1999).
Ye, S. et al. Enhanced cooperative quantum cutting in Tm3+-Yb3+ codoped glass ceramics containing LaF3 nanocrystals. Opt. Express 16, 8989–8994 (2008).
Chen, D. Q. et al. Quantum cutting downconversion by cooperative energy transfer from Ce3+ to Yb3+ in borate glasses. J. Appl. Phys. 104, 116105 (2008).
Roh, J. Y. D. et al. Negative thermal quenching in quantum-cutting Yb3+-doped CsPb(Cl1-xBrx)3 Perovskite nanocrystals. ACS Nano 17, 17190–17198 (2023).
Zi, L. et al. X-ray quantum cutting scintillator based on CsPbClxBr3-x: Yb3+ single crystals. Laser Photon. Rev. 17, 2200852 (2023).
Zhao, Y. Optical temperature sensing of up-conversion luminescent materials: fundamentals and progress. J. Alloys Compd 817, 152691 (2020).
Zhu, L. & Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: a review. Sens. Actuat. A: Phys. 267, 242–261 (2017).
Chen, Z. L., Galli, M. & Gallavotti, A. Mechanisms of temperature-regulated growth and thermotolerance in crop species. Curr. Opin. Plant Biol. 65, 102134 (2022).
de Mendívil, J. M. et al. Judd-Ofelt analysis and transition probabilities of Er3+ doped KY1-x-yGdxLuy(WO4)2 crystals. J. Lumin. 165, 153–158 (2015).
Zhang, L. Z. et al. Crystal growth, spectroscopy and first laser operation of a novel disordered tetragonal Tm: Na2La4(WO4)7 tungstate crystal. J. Lumin. 203, 676–682 (2018).
Zhang, L. Z. et al. Crystal growth, optical spectroscopy and laser action of Tm3+-doped monoclinic magnesium tungstate. Opt. Express 25, 3682–3693 (2017).
Auzel, F. A fundamental self-generated quenching center for lanthanide-doped high-purity solids. J. Lumin. 100, 125–130 (2002).
Liang, Y. J. et al. New function of the Yb3+ ion as an efficient emitter of persistent luminescence in the short-wave infrared. Light Sci. Appl. 5, e16124 (2016).
Zhang, Y. et al. Blue LED-pumped intense short-wave infrared luminescence based on Cr3+-Yb3+-co-doped phosphors. Light Sci. Appl. 11, 136 (2022).
Green, M. A. Solar Cells: Operating Principles, Technology, and System Applications (Kensington: University of New South Wales, 1998).
Zhang, Y. Q. et al. A universal approach for calculating the Judd–Ofelt parameters of RE3+ in powdered phosphors and its application for the β-NaYF4: Er3+/Yb3+ phosphor derived from auto-combustion-assisted fluoridation. Phys. Chem. Chem. Phys. 20, 15876–15883 (2018).
Luo, M. Y. et al. Optical transition properties, internal quantum efficiencies, and temperature sensing of Er3+ doped BaGd2O4 phosphor with low maximum phonon energy. J. Am. Ceram. Soc. 105, 3353–3363 (2022).
Sha, X. Z. et al. Pre-assessments of optical transition, gain performance and temperature sensing of Er3+ in NaLn(MoO4)2 (Ln = Y, La, Gd and Lu) single crystals by using their powder-formed samples derived from traditional solid state reaction. Opt. Laser Technol. 140, 107012 (2021).
de Sousa, D. F. et al. Energy transfer and the 2.8 - μm emission of Er3+-and Yb3+- doped low silica content calcium aluminate glasses. Phys. Rev. B 62, 3176–3180 (2000).
Pecoraro, E. et al. Evaluation of the energy transfer rate for the Yb3+: Pr3+ system in lead fluoroindogallate glasses. J. Appl. Phys. 86, 3144–3148 (1999).
Kim, M. et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 3, 2179–2192 (2019).
Zhou, T. M. et al. Concentration effect and temperature quenching of upconversion luminescence in BaGd2ZnO5: Er3+/Yb3+ phosphor. J. Rare Earths 33, 686–692 (2015).
Liu, L. T. et al. Dependence of optical temperature sensing and photo-thermal conversion on particle size and excitation wavelength in β-NaYF4: Yb3+, Er3+ nanoparticles. J. Alloys Compd 741, 927–936 (2018).
Li, Y. C. et al. A temperature self-monitoring NaYF4: Dy3+/Yb3+@NaYF4: Er3+/Yb3+ core-shell photothermal converter for photothermal therapy application. Results Phys. 15, 102704 (2019).
Collins, S. F. et al. Comparison of fluorescence-based temperature sensor schemes: theoretical analysis and experimental validation. J. Appl. Phys. 84, 4649–4654 (1998).
Acknowledgements
This work was partially supported by NSFC (National Natural Science Foundation of China, grant No. 12274049), Fundamental Research Funds for the Central Universities (grant No. 3132023519).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, D., Chen, B., Sha, X. et al. Near infrared emissions from both high efficient quantum cutting (173%) and nearly-pure-color upconversion in NaY(WO4)2:Er3+/Yb3+ with thermal management capability for silicon-based solar cells. Light Sci Appl 13, 17 (2024). https://doi.org/10.1038/s41377-023-01365-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41377-023-01365-2
This article is cited by
-
Multifunctional photon conversion materials for enhancing silicon solar cells
Light: Science & Applications (2024)
-
Ca3TeO6:Er3+, Yb3+ up-conversion phosphors for optical temperature sensor based on FIR technique
Applied Physics A (2024)