Abstract
Abnormal Drp1 activation and subsequent excessive mitochondrial fission play a critical role in ischemia-reperfusion injury (I/RI). Although fibroblast growth factor 21 (FGF21) protects organs against I/RI and regulates metabolism, which indicates that FGF21 is involved in mitochondria homeostasis, the detailed mechanism remains unclear. Herein, we investigated whether FGF21 had an effect on Drp1 activation during skeletal muscle I/RI. Drp1 phosphorylation and its translocation to mitochondria, as regulated by FGF21, was examined in mouse and C2C12 cell I/RI models. Mice overexpressing FGF21 displayed alleviation of serum index, histological lesions and apoptosis levels. Moreover, FGF21 markedly decreased cyclin-dependent kinase 1 (CDK1) and Drp1 phosphorylation at Ser616, accompanied by reduced accumulation in mitochondria. In parallel in vitro studies, cells with FGF21 knockdown displayed enhanced Drp1 activation, and the reverse effect was found when FGF21 was added. More importantly, FGF21 attenuated mitochondrial fission with linear mitochondria rather than fragmented mitochondria. Furthermore, a CDK1 inhibitor reduced Drp1 activation and mitochondrial fission due to FGF21 knockdown. This study shows that FGF21 inhibits Drp1 activation to protect mitochondria from fission, thereby rescuing cells from I/RI-induced apoptosis. Our findings may provide a new therapeutic approach to ameliorate skeletal muscle I/RI.
Similar content being viewed by others
Ischemia-reperfusion (I/R) injury is caused by the restoration of blood supply to tissues, and it aggravates the injury to the ischemic tissue1,2. It is well known that when I/R occurs in skeletal muscle followed by trauma, surgical operation, or embolism, a series of complications with adverse outcomes will severely affect patient quality of life3,4. Although some beneficial treatments have been explored, unfortunately, there are still no effective clinical therapies5,6. Therefore, it is necessary to explore the mechanism of I/R injury. Furthermore, developing more effective strategies to ameliorate I/R is also urgently required. Although the underlying molecular mechanisms of I/R have remained unclear until now, several crucial events such as oxidative stress, inflammation and apoptosis have been proposed to contribute to I/R injury7,8. Of note, apoptosis triggered by excessive mitochondrial fission has been considered as one of the most critical contributors to I/R injury9.
Dynamic-related protein-1 (Drp1) is the most important fission protein that mediates the dynamic process of mitochondrial fission10. Drp1 was mainly located in the cytosol, but upon activation, it is translocated to mitochondrial scission sites11. Furthermore, when Drp1 was phosphorylated at the Ser616 residue, mitochondrial fission occurred12. Fragmented mitochondria are unable to provide sufficient energy to cells and have been linked to cell apoptosis13,14, and thereby the following I/R injury. A large number of studies have shown that suppressing mitochondrial fission by inhibiting Drp1 activation could greatly mitigate apoptosis and alleviate I/R injury15,16. Activation of Drp1 is tightly regulated by protein kinase C (PKC) or cyclin-dependent kinase (CDK) 1/Cyclin B17,18.
Fibroblast growth factor 21 (FGF21) is mainly secreted by the liver but is also secreted by adipose and skeletal muscle19. FGF21 is dramatically elevated during some pathological conditions, while its levels are much lower under normal conditions20. FGF21 has beneficial metabolic effects on decreasing body weight, lowering glucose and lipid, increasing insulin sensitivity and energy expenditure, as well as inducing browning of white adipose tissue and adaptive thermogenesis. Besides, FGF21 is also a stress hormone which can combat oxidative stress and elicit an anti-inflammatory response, thereby exerting protective effects against NAFLD, NASH, cardiac diseases and pancreatitis. In addition, FGF21 increases life expectancy as well as regulates bone homeostasis21,22. Despite the fact that FGF21 can protect the heart and brain from I/R injury23,24, the exact mechanism is not completely understood. Many studies have proven the close relationship between FGF21 and metabolism, which mainly occurred in the mitochondria25,26. Consistently, studies also demonstrated that FGF21 can enhance mitochondrial function27,28. Collectively, all these data indicate us that FGF21 is tightly related to mitochondria; however, the underlying molecular mechanism by which FGF21 affects mitochondria warranted further study. It was reported that FGF21 regulated PKC in a dose-dependent manner29, while PKC is the upstream regulator of Drp1, consequently, these data indicate us that there is a great possibility that FGF21 regulate mitochondria fission though Drp1, so as to affect apoptosis and protect against I/R injury.
Therefore, we hypothesized that FGF21 could protect skeletal muscle from I/R injury through a novel functional role and regulatory mechanism on Drp1 activation. Our experiments showed that FGF21 can significantly inhibit Drp1 activation, mitochondria fission and consequent apoptosis by targeting CDK1 during skeletal muscle I/R. Our results identify a regulatory pathway involving FGF21 and Drp1 in skeletal muscle I/R injury.
Materials and methods
Animal model of skeletal muscle I/R
Male C57BL/6 mice aged 8 weeks received humane care following the NIH’s Guide for the Care and Use of Laboratory Animals approved by the Shandong University (Shandong, China). The mice were randomly divided into 6 groups: WT, WT + I/R, Ad-FGF21, Ad-FGF21 + I/R (1 × 1010 pfu FGF21-containing viral plasmid, intramuscular injection in situ), Ad-null, Ad-null+I/R (injection of vector control the same as Ad-FGF21 group). After 7 days of viral transfection, mice underwent I/R. Mice were wrapped with a rubber band around the groin for three circles to interrupt the blood supply. After 4 h of ischemia, the rubber band was removed and mice were sacrificed at 4 h of reperfusion.
Cell culture, RNA interference, and H/R
C2C12 myoblasts were incubated with Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 37 °C and 5% CO2. Differentiation was induced with 2% horse serum for 6 days.
The RNA interference technique was used for knockdown experiments. We have got three pairs of siRNA targeting mouse FGF21 mRNA (FGF21-siRNA767, 570, and 325) synthesized by GenePharma (Shanghai, China). Cell transfection was performed using LipofectamineTM RNAiMAX Transfection Reagent (Cat.# 1378030, Thermo), following the manufacturers’ instructions. The validity of the interference was examined by Western blotting.
After treated with FGF21 (100 ng/ml) for 24 h and transfection with siRNA for 48 h, cells were simulated with H/R. Cells were incubated in serum-free and no-glucose DMEM with a hypoxic treatment with 1% oxygen, 5% carbon dioxide, and 94% nitrogen for 5 h, then returned to conventional DMEM medium and normoxic environment for 2 h.
Antibodies and reagents
The antibodies applied in our experiments were as follows: FGF21 (Cat. # ab171941, Abcam); Drp1 (Cat.# ab219596, Abcam); phospho-Drp1 Ser616 (Cat.# 3455, Cell Signaling Technology); CDK1 (Cat.# 19532-1-AP, Proteintech); Bax (Cat.# 60267-1-Ig, Proteintech). Reagents in our study were purchased from the following suppliers: MitoTracker Deep Red (Cat.# M22426, Invitrogen); Recombinant mouse FGF21 (USCN Life Science), Minute™ Mitochondria Isolation Kit (Cat.# MP-007, Invent Biotechnologies), TUNEL assay kit (Cat.# C1090, Beyotime).
Skeletal muscle damage assessment serum
creatine kinase (sCK) and lactate dehydrogenase (sLDH) levels, indicators of skeletal muscle injury, were analysised by the clinical autoanalyzer (7600-020; Hitachi, Tokyo, Japan).
Quantitative real-time PCR
Total RNA was isolated and converted to cDNA using the ReverTra Ace (TRT-101; Toyobo, Osaka, Japan). Real-time quantitative PCRs were performed using SYBR Green PCR master mix (QPK201; Toyobo). Primers used in these analyses were as follows: FGF21, 5′-CTGCTGGGGGTCTACCAAG-3′ (forward) and 5′-CTGCGCCTACCACTGTTCC-3′ (reverse); Drp1, 5′-CAGGAATTGTTACGGTTCCCTAA-3′ (forward) and 5′-CCTGAATTAACTTGTCCCGTGA-3′(reverse).
Confocal microscopy
Mitochondria was labeled with MitoTracker. Cells or frozen sections were incubated with primary antibodies at 4 °C overnight, secondary antibody staining was 1 h at room temperature in an Alexa Fluor 488/594 IgG antibody solution. After nuclear staining with DAPI, staining was analyzed using a Laser Confocal Microscope (Leica TCS-NT SP8, Germany).
Statistical analysis
Results were reported as the average of at least three biological replications and expressed as means ± standard. The significance of between-group differences was tested by t-test and two-way analysis of variance.
Results
FGF21 alleviates skeletal muscle I/R injury in mice
To investigate whether FGF21 is protective against I/R injury in an in vivo model, mice were first transfected with FGF21-containing viral plasmids or vector by intramuscular injection in situ. The western blot results showed a robust increase in FGF21 protein expression in the skeletal muscle 7 days after administration of adenovirus to Ad-FGF21 mice compared with that in Ad-null mice. Endogenous FGF21 expression in skeletal muscle exposed to I/R was also analyzed. The I/R treatment obviously increased endogenous FGF21 expression, which indicated the involvement of FGF21 in I/R-induced skeletal muscle injury, but FGF21 in Ad-null mice was still much lower than that in Ad-FGF21 mice after I/R (Fig. 1A). Skeletal muscle from mice were subjected to 4 h of ischemia followed by 4 h of reperfusion.
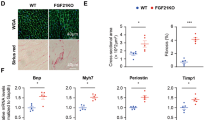
A FGF21 expression in skeletal muscle at 7 days after viral transfection and after I/R assayed by Western blot. B Skeletal muscle stained with hematoxylin and eosin. Severe swollen muscle fibers were indicated with arrows. C Serum CK and LDH levels. D ATP levels in skeletal muscle. E Western-assisted analysis and relative density ratio of Bax. F Immunofluorescence staining of Bax in skeletal muscle was detected by confocal microscopy. G TUNEL analysis of apoptosis. TUNEL-positive were red and DAPI were blue under fluorescence microscopy. Data represents mean ± SD of 8 animals in each group. *P < 0.05; **P < 0.01. Scale bars: 20 um (B); 100 um (F, G). WT, wild type; Ad-null, vector control viral transfection; Ad-FGF21, FGF21 viral transfection; CK creatine kinase, LDH, lactate dehydrogenase.
The histology showed that exposure to I/R induced significant skeletal muscle damage compared with that of the control group. Severe swollen and irregular muscle fibers and moderate inflammatory cell infiltration were observed in the Ad-null and WT mice that were subjected to I/R. However, FGF21-transfected mice showed markedly attenuated muscle fiber swelling and a more regular shape (Fig. 1B). Furthermore, serum CK and LDH levels, used as markers for the destruction of muscle fibers, were significantly increased in mice with I/R but decreased in Ad-FGF21 mice (Fig. 1C). Moreover, the ATP content was decreased after I/R injury, whereas it was better maintained in Ad-FGF21 mice (Fig. 1D). In addition, as another marker of liver injury, apoptosis was evaluated. I/R treatment clearly increased the level of Bax, whereas its elevation was prevented in the Ad-FGF21 mice (Fig. 1E). To further confirm our findings, Bax was also assessed by confocal laser microscopy. The red fluorescent spots representing Bax were significantly elevated in mice subjected to I/R, whereas FGF21 transfection dramatically inhibited this increase (Fig. 1F), which echoed the above western blot results. Moreover, considerable numbers of apoptotic TUNEL-positive cells were identified in the skeletal muscle slides from the Ad-null group compared with those from the Ad-FGF21 group after I/R (Fig. 1G). Collectively, these results strongly suggest that FGF21 inhibits apoptosis to combat I/R-induced skeletal muscle injury.
FGF21 inhibits Drp1 activation in skeletal muscle I/R injury in vivo
We then analyzed whether FGF21 regulates Drp1 activation in I/R-triggered skeletal muscle injury. Of note, the levels of Drp1 protein were apparently increased in association with I/R injury, whereas neither the Ad-null group nor the Ad-FGF21 group showed a difference in Drp1 expression at the protein level (Fig. 2A). This result suggests total Drp1 was unaffected by FGF21. To verify the role of FGF21 in regulating the translocation and phosphorylation of Drp1, mitochondrial and cytoplasmic fractions were isolated to detect the subcellular compartmentalization of Drp1. Phosphorylated Drp1 and mitochondrial-located Drp1 obviously increased with I/R treatment, whereas Ad-FGF21 exacerbated the increase. On the contrary, I/R resulted in a significant decrease in cytoplasmic Drp1 levels, but Ad-FGF21 prevented any further decline. Considering that Drp1 is regulated by CDK1, we detected the expression level of CDK1. I/R injury also resulted in a significant upregulation of CDK1, but the levels were robustly much lower in the Ad-FGF21 group compared with that in the Ad-null group (Fig. 2B). Furthermore, we’ve performed IHC staining to evaluate the expression of CDK1 and p-Drp1. There was no significant difference in the expression of CDK and p-Drp1 when mice were treated without the I/R injury. However, CDK1 and p-Drp1 expression (stained in brown) was obviously increased after the I/R damage while the intensity of CDK1, p-Drp1 was much weakened in Ad-FGF21 mice (Fig. 2C and Supplementary material). Together, these results suggest that FGF21 might inhibit Drp1 activation and translocation to mitochondria.
A Total Drp1 expression in skeletal muscle after I/R. B Analysis with Western blotting of effects of FGF21 on the phosphorylation of Drp1, CDK1 and distribution of Drp1 in Cytoplasmic (cyto) versus mitochondria (mito). C Immunohistochemistry staining of CDK1 and p-Drp1 in skeletal muscle. Data represents mean ± SD. *P < 0.05; **P < 0.01. Scale bars: 20 um (C). WT wild type, Ad-null, vector control viral transfection; Ad-FGF21, FGF21 viral transfection; p-Drp1, phosphorylated-Drp1 cyto cytosolic fraction, mito mitochondrial compartment.
Both FGF21 and activation of Drp1 are involved in H/R-induced C2C12 myoblasts injury
To further determine the relationship between FGF21 and Drp1, C2C12 myoblasts were used to generate an in vitro I/R model. We mimicked the I/R process with the hypoxia/reoxygenation (H/R) model in C2C12 cells. Cells were exposed to hypoxia for 1, 3, or 5 h and subsequently reoxygenated for 2 h. The result indicated that there was a distinct upregulation of FGF21 mRNA but a downregulation of Drp1 mRNA with H/R treatment. However, exposure to different hypoxia times did not affect their mRNA levels (Fig. 3A). At the same time, the FGF21 protein level was also increased after hypoxia for 5 h. Of note, the Drp1 level was almost unchanged, suggesting that total Drp1 may not be affected by H/R in C2C12 cells (Fig. 3B).
A Real-time quantitative PCR analysis of the mRNA level of FGF21 and Drp1. B Western blot analyses and density ratios of FGF21 and total Drp1. C Phosphorylation of Drp1 as well as cytoplasmic (cyto) versus mitochondria (mito) fractionation was analyzed to compare the distribution of Drp1. D ATP levels. E Accumulation of p-Drp1 (green) in mitochondria (red) by confocal microscopy. These experiments were repeated at least three times. Data represents mean ± SD. *P < 0.05; **P < 0.01. Scale bars: 100 um (E). p-Drp1 phosphorylated-Drp1; cyto cytosolic fraction, mito mitochondrial compartment.
Therefore, we further measured the phosphorylation levels and the consequent translocation of Drp1 to mitochondria by western blot analyses of cytoplasmic and mitochondrial proteins. Apparently, not only the phosphorylation of Drp1 but also the Drp1 levels in mitochondria were obviously increased, but Drp1 levels in the cytoplasm decreased with the H/R treatment in a time-dependent manner (Fig. 3C). Furthermore, the ATP content was reduced (Fig. 3D). To further verify our findings, C2C12 cells treated with H/R were subjected to confocal laser microscopy. As shown in Fig. 3E, in C2C12 cells that were not exposed to H/R, the morphology of the mitochondria was more tubular, linear or rod-like, while the green fluorescent puncta of p-Drp1 and its attachment to the periphery of mitochondria were almost not detected. However, H/R injury resulted in a sharp increase in the green fluorescent puncta; in addition, the accumulation of p-Drp1 in mitochondria was markedly enhanced, accompanied by the accelerated fragmentation of mitochondria with a more round and punctate appearance, whose peak levels were reached at 5 h of hypoxia. Consequently, exposure to hypoxia for 5 h and subsequent reoxygenation for 2 h was selected for further experiments. These results indicate that FGF21 and activation of Drp1 are involved in H/R-induced C2C12 cell injury and that there might be an interplay between them.
FGF21 inhibits the activation of Drp1 in H/R-induced C2C12 cell injury
To obtain the optimum conditions for the FGF21 regulation of Drp1, C2C12 cells were treated with various concentrations of FGF21 (50 ng/ml and 100 ng/ml) 24 h before H/R. Interestingly, the accumulation of Drp1 in mitochondria was inhibited with both concentrations, while 100 ng/ml was more effective (Fig. 4A). Correspondingly, C2C12 cells treated with 100 ng/ml FGF21 were used in the following experiment. Furthermore, the effect of FGF21 was also confirmed through a knockdown approach. We obtained three siRNAs targeting FGF21, and the validity of the interference was examined by western blotting. Transfection of these FGF21-siRNAs dramatically reduced the FGF21 protein content (Fig. 4B). As Fig. 4C indicates, after H/R treatment, the ATP content was significantly decreased; transfection of FGF21-siRNA enhanced the reduction, while treatment of cells with FGF21 alleviated the reduction. Moreover, H/R treatment also resulted in a significant increase in Bax in siFGF21 cells compared with siNC cells; in contrast, the increase in Bax was obviously much lower in FGF21-treated cells (Fig. 4D). These results suggested that FGF21 protects C2C12 cells from H/R injury.
A Effects of various concentrations FGF21 on Drp1 in mitochondria. B RNA interference effect on FGF21 expression. C Cellular ATP content. D Western blot analyses of Bax, Drp1 phosphorylation and translocation to mitochondrial after added with FGF21 or knockdown. E Immunofluorescence staining of p-Drp1 (green) in mitochondria (red). All data were repeated three times. Data represents mean ± SD. *P < 0.05; **P < 0.01. Scale bars: 100 um (E). siNC transfection with scrambled siRNA, siFGF21 transfection with FGF21-siRNA, p-Drp1 phosphorylated-Drp1, cyto cytosolic fraction, mito mitochondrial compartment.
We further detected the role of FGF21 in regulating Drp1 in vitro. As expected, the experimental results in vitro were highly consistent with the previous in vivo results. FGF21 knockdown cells showed enhanced phosphorylation of Drp1 and its accumulation in mitochondria, but Drp1 was decreased in the cytoplasm, and the reverse effect was found in cells treated with FGF21 (Fig. 4D). Immunofluorescence staining was also used to further assess the potential effects of FGF21 on the regulation of Drp1. As shown in Fig. 4E, compared with those of the control group, exposure to H/R enhanced fragmented mitochondria, green fluorescent puncta of p-Drp1 and its translocation to mitochondria, which were the most prominent in siFGF21 cells. In contrast, these phenomena were markedly suppressed in cells treated with FGF21. More importantly, linear or rod-like mitochondria rather than fragmented mitochondria were better maintained in the FGF21-treated cells than in the other two groups. Therefore, consistent with our previous findings in mice, these in vitro results confirm that FGF21 inhibits the activation of Drp1.
FGF21 inhibits Drp1 activation by suppressing CDK1
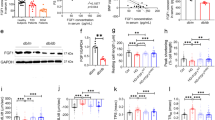
To gain insight into the underlying mechanism of how FGF21 regulates Drp1, we also tested whether CDK1 was altered because CDK1 has been reported to stimulate Drp1 activation30,31. Consistent with our in vivo results, H/R led to dramatic increases in the content of CDK1 protein; FGF21 knockdown cells showed significantly higher levels than siNC cells, while cells treated with FGF21 manifested the opposite results. Meanwhile, FGF21 concentration increased after ischemia reperfusion injury, but it was still lower in siFGF21 cells than in siNC cells. Besides, the expression of FGF21 was highest when cells treated with FGF21 (Fig. 5A).
A Western blot analyses of FGF21 regulating CDK1. B Drp1 phosphorylation and translocation to mitochondrial after inhibiting CDK1 in FGF21 knockdown C2C12 cells. C: Colocalization of p-Drp1 (green) and mitochondria (red). The assays were repeated at least three times. Data represents mean ± SD. *P < 0.05; **P < 0.01. Scale bars: 100 um (C). siNC, transfection with scrambled siRNA; siFGF21, transfection with FGF21-siRNA; p-Drp1 phosphorylated-Drp1, cyto cytosolic fraction, mito mitochondrial compartment.
We further sought to determine whether FGF21 regulates Drp1 through CDK1. Ro-3306, an inhibitor of CDK132,33, was added to suppress the increase of CDK1 in siFGF21 cells during H/R. Similarly, after ischemia reperfusion injury, enhanced Drp1 phosphorylation, decreased localization of Drp1 in the cytoplasm, as well as increased Drp1 in mitochondria were observed in siFGF21 cells comparing with siNC cells, but these phenomena appeared to be stopped by blockade of CDK1 with Ro-3306 in siFGF21 cells (Fig. 5B). Correspondingly, accelerated Drp1 activation after exposure to H/R, as evidenced by the Drp1 phosphorylation visualized by green fluorescence and its recruitment to mitochondria was weakened in the presence of Ro-3306. Treatment with Ro-3306 also resulted in more elongated mitochondria (Fig. 5C). Collectively, these findings suggested that the regulatory effect of FGF21 on Drp1 was at least in part related to CDK1.
Discussion
In this study, we demonstrated that (1) FGF21 alleviates apoptosis and protects skeletal muscle against I/R injury; (2) FGF21 inhibits Drp1 phosphorylation and its mitochondrial translocation; (3) FGF21 regulates Drp1 by decreasing the levels of CDK1. Our results highlight the importance of FGF21 in orchestrating Drp1 activation and its protective role in I/R-induced skeletal muscle injury. This study provides new evidence documenting the key role of FGF21 in the regulation of Drp1-mediated mitochondrial fission in skeletal muscle I/R injury.
I/R injury is one of the major reasons for failed transplantations, where excessive mitochondrial fission and apoptosis have been described34. Although there are increasing numbers of innovations aiming to elucidate strategies for mitigating I/R injury in skeletal muscle, and some methods have been widely used, it remains a challenge in clinical practice. Therefore, effective protectors are urgently required to alleviate I/R injury of skeletal muscle.
FGF21 undoubtedly has advantages in I/R protection over other growth factors owing to its protective effect. FGF21 has recently attracted great interest as a therapeutic agent for various I/R disease including cardiac and neurological diseases. In our study, we found that targeted intervention of FGF21 is therapeutically efficient in inhibiting skeletal muscle I/R injury. The protective effects of FGF21 were not only demonstrated in an Ad-FGF21 mouse model with high expression of FGF21 but were also demonstrated in C2C12 cells treated with FGF21 or knockdown.
Although the roles of apoptosis in skeletal muscle I/R injury have not been fully elucidated35, many studies have shown that targeting apoptosis is effective in inhibiting I/R. Previous studies have shown that the protective effect of FGF21 during neuronal I/R is achieved by attenuating apoptosis24; however, it was not clear if FGF21 is involved in the regulation of apoptosis and mitochondrial fission in skeletal muscle I/R damage. In our study, this hypothesis was confirmed by both in vitro and in vivo experiments. The overexpression of FGF21 reduced the expression of Bax and TUNEL-positive cells in mouse skeletal muscle that had undergone I/R. Furthermore, FGF21 reduced the Bax level and reversed the reduction in ATP in C2C12 cells after H/R treatment. Nevertheless, mitochondrial fragmentation is a potent trigger of apoptosis that leads to cell death. Fragmented mitochondria cannot produce energy, which in turn causes progressively greater damage to cells. FGF21 also inhibited abnormal mitochondrial morphology in the form of a spotted appearance, preserving a more linear mitochondrial morphology that allowed for the generation of more ATP after H/R. FGF21 alleviates mitochondrial fission and apoptosis, both of which help to preserve mitochondrial functionality and contribute to sufficient inhibition of cell damage. In support of other findings regarding its protective effect on the heart and brain, our study similarly suggests that FGF21 also plays a pivotal role in the protection of skeletal muscle against I/R injury, which is a brand new area of study. However, it is interesting to note that we found FGF21 mRNA level was increased but FGF21 protein took on a firstly decreasing and then increasing change after H/R in C2C12 cell. We suspect that, due to H/R injury which contributes to activation of many factors including FGF21 to combat the stress, thereby resulting in more consumption of FGF21 protein. In the early stage of injury, despite FGF21 mRNA level was augmented on account of the gene sensibility, nevertheless it could not promptly and sufficiently to make up for the loss of FGF21 protein, because the translation of protein lags behind the transcription. As consequence, we observed that the FGF21 protein was decreased. In addition, it is worth noting that although FGF21 protein was decreased comparing with normal conditions initially, it was gradually increased since 1 h after H/R, expression of FGF21 protein was enhanced ultimately at 5 h after H/R, which is identical to literatures that FGF21 is increased during some pathological conditions.
Drp1 is a master mediator of mitochondrial fission that aids in the maintenance of mitochondrial dynamic homeostasis36,37. Drp1 has been reported to be involved in the I/R process in the liver and heart, and inhibiting Drp1 activation to thereby attenuate mitochondrial fission and apoptosis is an effective approach to mitigate I/R injuries38,39. We found that FGF21 significantly weakened the activation of Drp1, at the same time, severe destruction of mitochondrial morphology was effectively abolished in cells treated with FGF21. All of these results suggest inhibition of Drp1 activation by FGF21 could reduce mitochondrial fission and consequently provide enough energy for cell survival and I/R injury relief. Furthermore, we observed the interesting discrepancy between Drp1 mRNA and protein. The amount of protein are not only affected by mRNA, but also regulated by many factors, such as post-translational modification. Drp1 is regulated by several post-translational modifications like small ubiquitin-like modification (SUMOylation), which is a common protein degradation pathway. Indeed, it was observed that Drp1 mRNA was downregulated after H/R, but it was probably hypothesized that except for change of Drp1 mRNA following H/R, compensatory Drp1 post-translational modification (SUMOylation) might be altered at the same time, facilitating attenuation of Drp1 degradation, accordingly maintenance of protein level. However, precious mechanism about the discrepancy between the mRNA and protein remains to be continuously explored.
Identification of the possible mechanism by which FGF21 regulates Drp1 activation in skeletal muscle I/R has encouraged further investigations into the mechanisms that have not been characterized to date. Previous studies have shown that Ca2+/calmodulin-dependent protein kinase Iα (CaMKIα), kinase II (CaMKII), and CDK1 promote the phosphorylation of Drp1 at Ser616 and increase Drp1 translocation to mitochondria40,41. In this study, we found that CDK1 was also regulated by FGF21. When combined with Ro-3306 to inhibit CDK1, the enhanced Drp1 activation and mitochondrial fission due to FGF21 knockdown were obviously weakened, suggesting that FGF21 inhibited Drp1 activity at least partly through CDK1. Further study is needed to identify the possible mechanism by which Drp1 is regulated by FGF21 during I/R in skeletal muscle.
Conclusion
In conclusion, our data demonstrate the tight relationship among FGF21, Drp1, CDK1, and apoptosis. Our study indicates that FGF21 plays an important role in regulating Drp1 activity and mitochondrial dynamics during skeletal muscle I/R (Fig. 6). Nevertheless, our findings provide the rationale for classifying FGF21 as a protector that may serve as a new therapeutic approach to ameliorate skeletal muscle I/R injury.
When skeletal muscle were underwent I/R injury, on the one hand, FGF21 as a stress regulator was upregulated; on the other hand, Drp1 activated by CDK1 was translocated to mitochondrial which exacerbating mitochondrial fission. The excessive fragmented mitochondria initiated apoptosis and the following cell injury. However, owning to upregulated FGF21, it can battle with Drp1 activation and consequently alleviate apoptosis induced by abnormal mitochondrial fission.
Data availability
Data supporting this study are available from the corresponding author upon reasonable request.
References
Kunecki, M., Płazak, W., Podolec, P. & Gołba, K. S. Effects of endogenous cardioprotective mechanisms on ischemia-reperfusion injury. Postȩpy higieny i Medycyny Doświadczalnej 71, 20–31 (2017).
Granger, D. N. & Kvietys, P. R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 6, 524–551 (2015).
Gillani, S., Cao, J., Suzuki, T. & Hak, D. J. The effect of ischemia reperfusion injury on skeletal muscle. Injury 43, 670–675 (2012).
Zhou, Y., et al. Age-dependent changes cooperatively impact skeletal muscle regeneration after compartment syndrome injury. Am. J. Pathol. 184, 2225–2236 (2014).
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Percival, T. J. & Rasmussen, T. E. Reperfusion strategies in the management of extremity vascular injury with ischaemia. Br. J. Surg. 99 Suppl 1, 66–74 (2012).
Odeh, M. The role of reperfusion-induced injury in the pathogenesis of the crush syndrome. New Engl. J. Med. 324, 1417–1422 (1991).
Kim, Y., et al. Early Treatment with Poly(ADP-Ribose) Polymerase-1 Inhibitor (JPI-289) reduces infarct volume and improves long-term behavior in an animal model of ischemic stroke. Mol. Neurobiol. 55, 7153–7163 (2018).
Wu, B., et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut 56, 645–654 (2007).
Wang, J. X., et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 17, 71–78 (2011).
Smirnova, E., Griparic, L., Shurland, D. L. & van der Bliek, A. M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245-2256 (2001).
Cribbs, J. T. & Strack, S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO reports 8, 939–944 (2007).
Tanaka, A. & Youle, R. J. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol. Cell 29, 409–410 (2008).
Frank, S., et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 (2001).
Karbowski, M., et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol., 159, 931-938 (2002).
Ong, S. B., et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121, 2012–2022 (2010).
Zaja, I.,et al. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem. Biophys. Res. Commun. 453, 710–721 (2014).
Taguchi, N., Ishihara, N., Jofuku, A., Oka, T. & Mihara, K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 282, 11521–11529 (2007).
Li, H., Zhang, J. & Jia, W. Fibroblast growth factor 21: a novel metabolic regulator from pharmacology to physiology. Front. Med. 7, 25–30 (2013).
Badman, M. K., Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437 (2007).
Fisher, F. M. & Maratos-Flier, E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 78, 223–241 (2016).
Geng, L., Lam, K. & Xu, A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 16, 654–667 (2020).
Hu, S., Cao, S., Tong, Z. & Liu, J. FGF21 protects myocardial ischemia-reperfusion injury through reduction of miR-145-mediated autophagy. Am. J. Transl. Res. 10, 3677-3688 (2018).
Ye, L., et al. FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho. Exp. Neurol. 317, 34–50 (2019).
Kharitonenkov, A., et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 115, 1627–1635 (2005).
Cuevas-Ramos, D., The role of fibroblast growth factor 21 (FGF21) on energy balance, glucose and lipid metabolism. Curr. Diabetes Rev. 5, 216–220 (2009).
Suomalainen, A., et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. The Lancet. Neurology 10, 806–818 (2011).
Ji, K., et al. Skeletal muscle increases FGF21 expression in mitochondrial disorders to compensate for energy metabolic insufficiency by activating the mTOR-YY1-PGC1α pathway. Free Rad. Biol. Med. 84, 161–170 (2015).
Lee, M. S., et al. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-κB. Metab. Clin Exp. 61, 1142–1151 (2012).
Horbay, R. & Bilyy, R. Mitochondrial dynamics during cell cycling. Apoptosis 21, 1327-1335 (2016).
Hu, C., Huang, Y. & Li, L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int. J. Mol. Sci. 18, (2017).
Vassilev, L. T., et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl Acad. Sci. USA 103, 10660–10665 (2006).
Huang, J., Xie, P., Dong, Y., An, W. Inhibition of Drp1 SUMOylation by ALR protects the liver from ischemia-reperfusion injury. Cell Death Differ. 28, 1174–1192 (2021).
Maneechote, C., Palee, S., Chattipakorn, S. C. & Chattipakorn, N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J. Cell. Mol. Med. 21, 2643–2653 (2017).
Castellaneta, A., et al. Plasmacytoid dendritic cell-derived IFN-α promotes murine liver ischemia/reperfusion injury by induction of hepatocyte IRF-1. Hepatology 60, 267–277 (2014).
Mishra, P. & Chan, D. C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nature reviews. Mol. Biol. 15, 634–646 (2014).
Fonseca, T. B., Sánchez-Guerrero, Á., Milosevic, I. & Raimundo, N. Mitochondrial fission requires DRP1 but not dynamins. Nature 570, E34-E42 (2019).
Bi, J., et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 20, 296–306 (2019).
Wu, D., et al. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: Therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J.: Offic. Publ. Fed. Am. Soc. Exp. Biol. 34, 1447–1464 (2020).
Xu, S., et al. CaMKII induces permeability transition through Drp1 phosphorylation during chronic β-AR stimulation. Nat. Commun. 7, 13189 (2016).
Han, X. J., et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 182, 573–585 (2008).
Author information
Authors and Affiliations
Contributions
L.L.M. designed research and carried out experiments. L.B.X. analyzed data and prepared the figures. L.L.M. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Qilu Hospital (Qingdao), Shandong University ethics licensing committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, B., Liu, L. Fibroblast growth factor 21, a stress regulator, inhibits Drp1 activation to alleviate skeletal muscle ischemia/reperfusion injury. Lab Invest 102, 979–988 (2022). https://doi.org/10.1038/s41374-022-00787-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-022-00787-7
This article is cited by
-
The role of mitochondrial dynamics and mitophagy in skeletal muscle atrophy: from molecular mechanisms to therapeutic insights
Cellular & Molecular Biology Letters (2024)
-
FGF21 Inhibits Hypoxia/Reoxygenation-induced Renal Tubular Epithelial Cell Injury by Regulating the PPARγ/NF-κB Signaling Pathway
Cell Biochemistry and Biophysics (2024)
-
Development of muscle weakness in a mouse model of critical illness: does fibroblast growth factor 21 play a role?
Skeletal Muscle (2023)
-
FGF21-FGFR1 controls mitochondrial homeostasis in cardiomyocytes by modulating the degradation of OPA1
Cell Death & Disease (2023)