Abstract
Increased permeability and growth (angiogenesis) of blood vessels play a key role in joint swelling and pannus formation in inflammatory arthritis, a family of diseases influenced by reproductive hormones. The hormone prolactin (PRL) protects against joint inflammation, pannus formation, and bone destruction in adjuvant-induced arthritis and these effects may involve its proteolytic conversion to vasoinhibin, a PRL fragment that inhibits angiogenesis and vasopermeability. Here, we show that the intra-articular injection of an adeno-associated virus type-2 (AAV2) vector encoding vasoinhibin reduced joint inflammation, the hyperplasia, vascular density, and vasopermeability of the pannus, and the loss of bone in mice subjected to antigen-induced arthritis. In agreement, the AAV2 vasoinhibin vector reduced the expression of proinflammatory cytokines (interleukin-1β, interleukin-6), an endothelial cell marker (platelet endothelial cell-adhesion molecule 1), and proangiogenic molecules [vascular endothelial growth factor (VEGF), VEGF receptor 2, and hypoxia-inducible factor 1α] in the arthritic joint. Also, vasoinhibin reduced the synovial vasopermeability induced by the intra-articular injection of VEGF in healthy mice. Finally, vasoinhibin signals by blocking the phosphorylation/activation of endothelial nitric oxide synthase (eNOS) at Ser1179 and the AAV2 vasoinhibin vector inhibited the enhanced phosphorylation of eNOS Ser1179 in the arthritic joint. We conclude that vasoinhibin reduces joint inflammation and bone loss in arthritis by inhibiting pannus angiogenesis and vasopermeability via the blockage of VEGF-induced eNOS activation. These findings suggest the potential therapeutic benefit of AAV2-mediated vasoinhibin gene delivery in arthritis.
Similar content being viewed by others
Introduction
The term inflammatory arthritis defines a family of diseases, including rheumatoid arthritis (RA), where genetic, environmental, and sex factors drive joint inflammation. In RA, infiltration of immune cells and synovial cell proliferation result in the formation of an invading inflammatory tissue or pannus that destroys the cartilage and bone [1]. Pannus formation requires of new blood vessels (angiogenesis) to cope with the increased requirement of oxygen and nutrients and the delivery of inflammatory cells and molecules [2]. Also, the newly formed blood vessels are highly permeable, which facilitates edema formation and joint swelling [3]. Hence, factors able to inhibit angiogenesis and vasopermeability are a promising therapy in RA [4]. One such factor is prolactin (PRL), the hormone essential for lactation.
The female preponderance and the influence of reproductive states have long linked PRL to RA [5, 6]. PRL is a sexually dimorphic, reproductive hormone that increases in the circulation of some patients with RA and has actions on immune cells and joint tissues that can affect the progression of the disease [6]. Both negative and positive outcomes have been associated to PRL in the clinic and the conflicting findings may depend on circulating PRL levels, local synthesis of PRL by joint tissues, and complex interactions at the inflammatory milieu [6]. For example, the inflammatory milieu may promote the cleavage of PRL to vasoinhibin, a PRL fragment that inhibits angiogenesis and vasopermeability [7]. Matrix metalloproteases (MMP) are upregulated in the joints of patients with RA [8], proinflammatory cytokines stimulate MMP production by RA synovial cells [9], and MMP produced by joint tissues cleave PRL to vasoinhibin [10]. Recent pre-clinical work showed that PRL reduces joint inflammation, pannus formation, cartilage destruction, and bone loss in adjuvant-induced arthritis [11, 12]. These findings, together with the upregulation of MMP in the arthritic joint, raise the possibility that an enhanced generation of vasoinhibin, by virtue of its inhibitory effects on blood vessels, contributes to the protective effect of PRL in inflammatory arthritis.
The use of adenoassociated virus (AAV) vectors is one of the most promising gene therapy strategies for RA in terms of efficacy and safety issues [13]. These vectors have the advantages of sustained transgene expression, reduced potential for host-immune responses, and the capacity to transduce both dividing and non-dividing cells [14]. The subtype 2 vector (AAV2) is used in human clinical trials for arthritis [15] and has been successful for the delivery of antiangiogenic therapy in collagen-induced arthritis [16]. Here, we used the intra-articular delivery of vasoinhibin via an AAV2 vector to study its effects on joint inflammation, bone loss, and pannus angiogenesis and vasopermeability in mice subjected to antigen-induced arthritis, another recognized model of RA [17].
Materials and methods
Production of AAV2 vectors
Human vasoinhibin cDNA (codons 1–142 of human PRL) was cloned into a self-complementary AAV2 vector downstream of a cytomegalovirus immediate early promoter as previously described [18]. An AAV2 null vector that contains two AAV inverted terminal repeat sequences without any expression unit between was provided by Sanofi Genzyme Corporate (Cambridge, MA). Recombinant AAV2 vectors were generated in HEK-293 cells (ATCC, Manassas, VA) by transient transfection with the triple-plasmid system and purified by polyethylene glycol precipitation and cesium chloride gradient, using a previously described method [19]. The viral vector quantification was done by RT-PCR comparing its DNA signal with a plasmid DNA standard curve. The AAV2 vectors were stored at −80 °C before use. A suspension of 10 µL containing 6.7 × 109 vector genomes (vg) of each vector was injected into the intra-articular space of knee joints one day before starting or not the induction of arthritis.
Animals
Male C57BL6 mice (8 weeks old, 20–25 g), were housed under standard laboratory conditions (22 °C, 12 h/12-h light/dark cycle, and free access to food and water) and cared for in accordance with the US National Research Council’s Guide for the Care and Use of Laboratory Animals (Eight Edition, National Academy Press, Washington, D.C., USA). The Bioethics Committee of the Institute of Neurobiology of the National University of Mexico (UNAM) approved all animal experiments.
Induction of antigen-induced arthritis (AIA)
In contrast to other polyarthritis models (collagen-induced arthritis and adjuvant-induced arthritis), AIA is monoarticular, i.e., it remains confined to the antigen-injected joint, enabling the use of the more accessible knee joint compared with ankles [20]. AIA was induced as described [21]. Briefly, two intradermal injections of 20 µL containing methylated bovine serum albumin (mBSA, 10 μg/μL, Sigma, St. Louis, MO) emulsified with an equal volume of complete Freund’s adjuvant (CFA, 5 μg/μL, Difco Laboratories, Detroit, MI) were delivered at the base of the tail on days 0 and 7. Mice also received an intraperitoneal injection of 10 µL of Bordetella pertussis toxin (10 ng/µL; Sigma, St. Louis, MO) at both time points to enhance the immune response. On day 21, mice were injected into the knee joint cavity with 10 µL of mBSA (2 µg/µL). Knee joint swelling was monitored by two observers blind to the experiment at defined time points during the course of AIA until day 27, when the animals were euthanized by CO2 inhalation and decapitation. Knee joints were processed for histological, qRT-PCR, and Western blot evaluation.
Pannus area and cellular density
Knee joints were fixed, decalcified, and dehydrated for paraffin embedding. Three-μm-thick sections of the mouse femur/tibia joint were stained with Harris’s hematoxylin-eosin solution for the evaluation of pannus area and nuclear density. Pannus area was traced and quantified relative to a fixed femur/tibia area using the Image-Pro Plus analysis software (Version 7, Media Cybernetics Inc.). The cellular density of the synovial hyperplasia was evaluated by quantifying the nuclear stain per area (Image-Pro Plus; Media Cybernetics Inc.).
Bone area and osteoclast density
Seven-μm-thick sections of the mouse femur/tibia joint were stained with tartrate-resistant acid phosphatase (TRAP) and counterstained with Weigert’s iron hematoxylin (Sigma) to evaluate the distal femur (500 μm below the growth plate) trabecular and cortical bone area and osteoclast density as previously reported [12]. Cortical and trabecular bone area and number of TRAP-stained magenta multinucleated cells (osteoclasts) were quantified (Image-Pro Plus analysis) in a blinded manner. The trabecular and cortical bone areas were measured per mm2 of bone. The number of osteoclasts was divided by the bone area (trabecular or cortical) to obtain the osteoclast density.
Vascular density
The vascular density of the synovial and pannus tissues was measured by immunostaining the blood vessels with an antibody against the endothelial cell marker, platelet endothelial cell-adhesion molecule 1 or CD-31 [22]. Briefly, 3-μm-thick paraffin sections were deparaffinized, rehydrated, and permeabilized with Tris/EDTA buffer (10 mM Tris-Base, 1 mM EDTA, 0.05% Tween 20, pH 9) for 1 min at 95–100 °C. Sections were incubated with a polyclonal antibody anti-CD31 (Abcam, Cambridge, Britain, 1:2000) and the reaction developed by the avidin-biotin peroxidase technique (Vectastain Elite ABC kit, Vector Laboratories) counterstained with hematoxylin & eosin stain. The density of the vascular network was determined by measuring the area positive for CD31 relative to the total area of the synovial membrane or pannus. Thin-walled vessels having a lumen size ≥10 µm were counted using the Image-ProPlus analysis software.
Vasopermeability
The vasopermeability of the synovium and pannus was evaluated by the Evans blue method with modifications [23]. Briefly, mice anesthetized using a mixture of 70% ketamine and 30% xylazine were injected in the lateral tail vein with the Evans blue stain (45 mg/kg, Sigma-Aldrich). The Evans blue was allowed to circulate for 2 h and then 300 μL of blood were collected by cardiac puncture to measure the tracer concentration in plasma. Mice were perfused for 2 min through the left ventricle with PBS (pH 3.5 at 37 °C). The synovial membrane or pannus tissues were dissected from the femoral-tibial joint and vacuum-dried (SPD 1010 SpeedVac System, ThermoSavant) for 5 h. After weighing the tissue, the tracer was extracted by incubating each sample in 100 μL of formamide (Sigma-Aldrich) for 18 h at 72 °C. The extract was centrifuged at 12,000 × g for 60 min at 4 °C and the supernatant absorbance was measured at 620 nm using the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). The Evans Blue tracer was quantified using a standard curve and values were normalized according to the tissue and body weight and the Evans blue concentration in plasma. To determine the effect of vasoinhibin on vascular endothelial cell growth factor (VEGF)-induced synovial membrane vasopermeability, healthy mice were injected into the femorotibial joint cavity with 15 µL of a isotonic saline solution containing 6 µl of VEGF (50 ng/µL; rhVEGF165; a gift from Genentech, South San Francisco, CA) with or without 8 µL of recombinant vasoinhibin (100 ng/µL). Recombinant human vasoinhibin (corresponding to a 14-kDa fragment of PRL) was generated by site-directed mutagenesis as previously described [24]. One day after intra-articular injections, the vasopermeability of the synovial membrane was evaluated using the Evans blue dye method as described above.
Quantitative real time-PCR (qRT-PCR)
Total RNA isolated from knee joints using TRIzol reagent (Invitrogen, Carlsbad, CA) was reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and diluted (10 ng/µL) in RNase-free water. Gene expression analysis by quantitative RT-PCR was done using Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA) in a final reaction of 10 μL containing 5 µL of sample cDNA and 0.5 μM of each of the following primer pairs for human vasoinhibin: foward 5′-CTGCCCGATGCCAGGTGA-3′ and reverse 5′-GAAAGTCTTTTTGATTCATCTGT-3′; and for the mouse genes: Pecam1: forward 5′-ATGACCCAGCAACATTCACA-3′ and reverse 5′- TCGACAGGATGGAAATCACA-3′; Hif1 α: forward 5′-ACAAGTCACCACAGGACAG-3′ and reverse 5′- AGGGAGAAAATCAAGTCG-3′; Vegfa: forward 5′- CGCGAGTCTGTGTTTTTGCA-3′ and reverse 5′- CAGAGCGGAGAAAGCATTTGT-3′; Vegfr2: forward 5′- GCCCTGCCTGTGGTCTCACTAC-3′ and reverse 5′- CAAAGCATTGCCCATTCGAT-3′; Il1b: forward 5′-GTTGATTCAAGGGGACATTA-3′ and reverse 5′-AGCTTCAATGAAAGACCTCA-3′; Il6: forward 5′-GAGGATACCACTCCCAACAGACC-3′ and reverse 5′-AAGTGCATCATCGTTGTTCATACA-3′; and Icam1: forward 5′- GCTGGGATTCACCTCAAGAA-3′ and reverse 5′- TGGGGACACCTTTTAGCATC-3′. qRT-PCR reactions were incubated for 10 min at 95 °C and 10 s at 95 °C, 30 s at the primer pair-specific annealing temperature and 30 s at 72 °C for 35 cycles. The mRNA expression levels of the gene of interest were calculated using the 2-ΔΔCT method and normalized to the housekeeping gene hprt.
Western blot analysis of eNOS phosphorylation
Six days after the intra-articular injection of mBSA, the knee joints were dissected, pulverized in liquid nitrogen using a mortar and pestle, and homogenized in lysis buffer (50 mM Tris Base, 150 mM NaCl, 0.5% Igepal, 0.1% SDS, pH 7.5) with 1/100 (v/v) of a protease inhibitor cocktail (P8340; Sigma, St. Louis, MO). Sixty µg of total protein was subjected to reducing SDS/PAGE (7.5% polyacrylamide gels), blotted, and probed overnight with a 1:500 dilution of anti-p-eNOS Ser1179 (9572; Cell Signaling, Beverly, MA, USA), anti-eNOS (9572; Cell Signaling), and a 1:1000 dilution of anti-β-Tubulin (ab6046; Abcam, Cambridge, MA) primary antibodies. Secondary antibodies conjugated to alkaline phosphatase (1:5000) or to horseradish peroxidase (1:5000) (both from Jackson ImmunoResearch Laboratories Inc., West Grove, PA) were used. Densitometry analysis was performed using the ImageJ Software (Bio-Rad, Richmond, CA).
Statistical analysis
Statistical analysis was performed using the Sigma Stat 7.0 software (Systat Sofware, San Jose, CA). Normality and equality of variances were determined by the D’Agostino–Pearson test and Levene’s test, respectively. Statistical differences between 2 groups was determined by the Student’s t test and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc compared means of multiple groups. All results were expressed as mean ± SEM. The threshold for significance was set at P < 0.05.
Results
Verification of vasoinhibin transgene expression in the knee joint
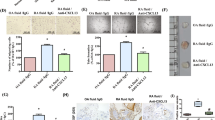
The expression of the vasoinhibin transgene was examined by qRT-PCR in femoral-tibial joints of non-arthritic and arthritic mice four weeks after the intra-articular injection of the AAV2 vasoinhibin vector or the AAV2 null vector. The human vasoinhibin transcript was found in joints injected with the vector encoding vasoinhibin but not with the null vector (Fig. 1a), confirming that the AAV2 vasoinhibin vector mediates vasoinhibin expression and that the primers used do not amplify endogenous mouse PRL. The expression of the vasoinhibin transgene appeared higher in the non-arthritic joint, although the difference was not statistically significant.
a. Quantitative RT-PCR analysis of vasoinhibin (Vi) mRNA levels in knee joints obtained from mice four weeks after they had been injected into the knee joint with AAV2-Vi or AAV2-null vectors. Induction (AIA) or not (Control) of AIA began one day after vector administration. Values are means ± SEM. Numbers inside bars indicate the number of joints. b. Time course of knee swelling as evaluated by the knee circumference one day before (day −1) the intra-articular injection of methylated bovine serum albumin (mBSA; day 0) and at the following days 1, 3, and 5. Values are means ± SEM (n = 10–12). *P < 0.05 vs. AIA AAV2-null.
The AAV2 vasoinhibin vector reduces the inflammation, pannus area, and bone loss of the arthritic joint
To investigate the effect of vasoinhibin on joint inflammation, the circumference of the knee joint was measured as an index of joint swelling one day before (day −1) the intra-articular injection of mBSA (day 0) and at the following days 1, 3, and 5. Treatment with the AAV2 vasoinhibin vector reduced joint swelling relative to the mice injected with the null vector at all time points post-mBSA injection (Fig. 1b). None of the vectors modified the joint circumference in non-arthritic mice.
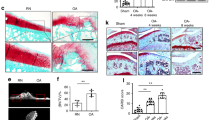
Histological examination of arthritic knee joints showed a severe disease characterized by extensive pannus formation, cartilage degradation, and bone erosion (Fig. 2a). Inflammatory changes were ameliorated by the treatment with the AAV2 vasoinhibin vector as verified by the significant reduction in the pannus area relative to the joint area. None of the vectors altered the healthy knee joint (Fig. 2a). Bone loss was further investigated by evaluating the trabecular and cortical bone areas and the density of TRAP-positive multinucleated cells (osteoclasts) in distal femur of tibiofemoral joint sections from AAV2-null vector and AAV2 vasoinhibin vector-injected mice. Trabecular and cortical bone areas were reduced and osteoclast density enhanced in arthritic joints injected with the AAV2-null vector compared with the null vector-injected nonarthritic joint (new Fig. 2b). Treatment with the AAV2 vasoinhibin vector prevented the loss in trabecular and cortical bone and reduced the increase in osteoclast density of arthritic joints. None of the vectors modified bone area and osteoclast density in none arthritic joints.
a Representative micrographs of the knee joint of healthy (control) and AIA mice injected with the AAV2 vasoinhibin vector (AAV2-Vi) or the AAV2-null vector. Sections were stained with hematoxylin and eosin. Cartilage degradation and bone erosion are indicated (arrows). sm synovial membrane, c cartilage, p pannus. Scale bar = 500 µm. The graph shows the synovial membrane or pannus area relative to a fixed area of the femorotibial joint. b Representative micrographs of tartrate-resistant alkaline phosphatase (TRAP)-stained sections of the femorotibial joint where TRAP-positive magenta multinucleated cells (osteoclasts) are indicated (arrows) in the cortical and trabecular bone areas. Inserts magnify an osteoclast. Graphs show trabecular and cortical bone areas and density of osteoclasts per each bone area. ct cortical bone, tb trabecular bone. Scale bar 500 μm. c Representative micrographs of the pannus and synovial membrane of AIA and control mice injected with the AAV2 vectors and stained with hematoxylin and eosin. The graph shows the cellular density values quantified by the nuclear stain per area of synovial membrane and pannus. Scale bar = 100 µm. Numbers inside bars indicate the number of joints, synovial membrane or pannus evaluated from independent animals. Values are means ± SEM. n.s. non-significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Pannus hyperplasia was evaluated by quantifying nuclear staining per area as an index of cellular density (Fig. 2c). Cellular density increased by ∼5-fold in arthritic joints injected with the null vector relative to the non-arthritic condition and was significantly reduced after treatment with the AAV2 vasoinhibin vector. The reduction in pannus hyperplasia may involve the lower influx of leukocytes into the joint. Consistent with this possibility, the expression levels of the gene encoding for intercellular adhesion molecule-1 (Icam1), a cell-adhesion molecule expressed in endothelial cells that promotes adhesion and transmigration of circulating leukocytes [25], increased in arthritic joints injected with the null vector and was significantly reduced by treatment with AAV2 vasoinhibin vector (Fig. 3). These findings indicate that local treatment with AAV2 vasoinhibin vector results in the reduction of joint inflammation, pannus formation, and bone loss in arthritis.
Quantitative RT-PCR-based quantification of the expression of the genes encoding the intercellular adhesion molecule 1 (Icam1), the endothelial cell marker, platelet endothelial cell-adhesion molecule 1 or CD-31 (Pecam1), and the proangiogenic molecules: hypoxia-inducible factor 1α (Hif1a), vascular endothelial growth factor (Vegf), receptor type 2 of VEGF (Vegfr2), interleukin 1β (Il1b) and interleukin 6 (Il6) in joints of non-arthritic (Control) or AIA mice injected with the AAV2 vasoinhibin (AAV2-Vi) or AAV2-null vectors. Values are means ± SEM. Numbers inside bars indicate the number of joints evaluated from independent animals. n.s. non-significant, *P < 0.05, **P < 0.01, ***P < 0.001.
The AAV2 vasoinhibin vector reduces the expression of angiogenesis and proinflammatory markers in the knee joint of arthritic mice
Angiogenesis is a key event in joint inflammation as it is required for the formation and maintenance of the pannus [26]. Because vasoinhibin inhibits angiogenesis [7], we reasoned that the AAV2 vasoinhibin vector could be ameliorating the disease by targeting synovial angiogenesis. Consistent with this possibility, joints transduced with the AAV2 vasoinhibin vector showed a significant reduction of the arthritis-induced expression of genes encoding the endothelial cell marker platelet endothelial cell-adhesion molecule 1 or CD-31 (Pecam 1) and the pro-angiogenesis molecules: vascular endothelial growth factor (VEGF, Vegf), VEGF receptor type 2 (Vegfr2), and hypoxia-inducible factor 1α (Hif1a) (Fig. 3). Also, treatment with the AAV2 vasoinhibin vector reduced the expression due to arthritits of the genes Il1b and Il6 that encode for the proinflammatory cytokines interleukin 1β (IL-1β) and interleukin 6 (IL-6) also known to be proangiogenic [27, 28] (Fig. 3).
The AAV2 vasoinhibin vector reduces the vascular density of the pannus
The downregulation of proangiogenic mediators (Pecam1, Vegfr2, Vegf, and Hif1α) implied the disruption of joint angiogenesis by the AAV2 vasoinhibin vector. To verify this implication, the vascular density and the number of blood vessels in the pannus and synovial tissue were assessed by immunohistochemistry against CD31 in knee joints from arthritic and healthy mice, respectively (Fig. 4a). No vascular change was observed in the joints of healthy mice injected with either vector. However, the vascular density and number of vessels increased ∼5- and ∼2-fold, respectively, in the pannus of arthritic mice treated with the AAV2 null vector and were significantly reduced by the vasoinhibin transduction with the AAV2 vector. These findings were confirmed after evaluating whole pannus dissected from the joint of arthritic mice where both, vascular density and number of vessels, were reduced after treatment with the AAV2 vasoinhibin vector relative to the null vector (Fig. 4b).
Representative micrographs of the pannus and synovial membrane in knee joints (a) or isolated from knee joints (b) from healthy (control) or AIA mice injected with the AAV2 vasoinhibin (AAV2-Vi) or AAV2-null vectors. Sections were immunostained with antibodies against the endothelial cell marker, platelet endothelial cell-adhesion molecule 1 or CD-31 (brown) and counterstained with hematoxylin and eosin. Some vessels are indicated (arrows). Inserts magnify a vessel. Scale bar = 100 µm. Vascular density in A and B was determined by quantifying the area stained for blood vessels relative to the total area of the tissue. Numbers inside bars indicate the number of joints or isolated synovial or pannus tissues evaluated. Values are means ± SEM. n.s, not significant; **P < 0.01, ***P < 0.001.
Vasoinhibin reduces the increase in synovial vasopermeability in response to arthritis and VEGF
Vasopermeability increases joint swelling in RA [29] and vasoinhibin reduces retinal vasopermeability in diabetic rats [18, 23]. To investigate whether reduction of vasopermeability contributes to the protective effect of the AAV2 vasoinhibin vector on joint inflammation, we measured the accumulation of Evans blue-linked albumin in the knee joints of arthritic and non-arthritic mice (Fig. 5a). There was a significant (∼10-fold) increase in tracer accumulation in arthritic joints injected with the AAV2-null vector and this increase was significantly reduced by the treatment with the AAV2 vasoinhibin vector. None of the vectors modified tracer accumulation in non-arthritic joints. VEGF is a major vascular permeability factor in RA [26] and vasoinhibin inhibits VEGF-induced increase in retinal vasopermeability [18, 30]. To assess whether vasoinhibin reduces VEGF-induced increase in synovial vasopermeability, VEGF was injected into the knee cavity of healthy mice alone or in combination with recombinant vasoinhibin and the synovial membrane was extracted 24 h after injection to evaluate its vasopermeability by the Evans blue method (Fig. 5b). VEGF caused a significant increase in the synovial accumulation of Evans blue-linked albumin that was prevented by its co-injection with vasoinhibin. Vasoinhibin by itself did not modify synovial vasopermeability. These findings suggest that vasoinhibin protects against joint inflammation in arthritis by targeting the vasopermeability effect of VEGF.
a Vascular permeability evaluated by quantifying the Evans blue-stained albumin extravasated into the synovial or pannus tissues from non-arthritic (control) mice or mice subjected to antigen-induced arthritis (AIA) and injected with AAV2 vasoinhibin (AAV2-Vi) or AAV2-null vectors. b Vascular permeability evaluated by quantifying the Evans blue-stained albumin extravasated into the synovial membrane of healthy mice 24 h after the intra-articular injection of VEGF (VEGF) in combination with recombinant vasoinhibin (Vi) or vehicle (Veh). Values are means ± SEM. Numbers inside bars indicate the number of synovial or pannus tissues evaluated. n.s. non-significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Vasoinhibin inhibits the phosphorylation/activation of endothelial nitric oxide synthase in the arthritic joint
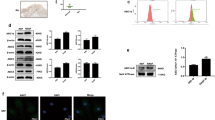
Vasoinhibin inhibits the vascular effects of VEGF by mechanisms including blockage of VEGF-induced phosphorylation/activation of endothelial nitric oxide synthase (eNOS) at Ser1179 [7, 30]. To investigate whether this mechanism operates in the arthritic joint, we evaluated eNOS phosphorylation by Western blot in knee joints from arthritic and non-arthritic mice treated with the AAV2 vasoinhibin and null vectors (Fig. 6). Arthritis enhanced eNOS phosphorylation at Ser1179 as compared with the non-arthritic condition in joints treated with the null vector, and this effect was not observed in arthritic joints injected with the AAV2 vasoinhibin vector. The differences were significant after quantifying phosphorylated eNOS normalized for the amount of β-tubulin (Fig. 6) or total eNOS (not shown) on the gel. These results suggest that vasoinhibin signals in arthritis by blocking VEGF-induced eNOS activation.
Representative Western blot analysis of eNOS-Ser1179 phosphorylation in joints from non-arthritic mice or mice subjected to antigen-induced arthritis (AIA) injected with the AAV2 vasoinhibin (AAV2-Vi) or AAV2-null vectors. Total eNOS and β-tubulin served as loading controls. Graphs show the quantitation of eNOS phosphorylation by densitometry after normalization for β-tubulin. Values are means ± SEM. Numbers inside bars indicate the number of knee joints evaluated from independent animals. n.s. non-significant, **P < 0.01.
Discussion
The PRL/vasoinhibin axis is a recently defined endocrine axis where the generation of vasoinhibin is regulated at the hypothalamus, the pituitary, and the target tissue levels [31]. The PRL/vasoinhibin axis regulates the growth and function of reproductive and non-reproductive organs and contributes to the pathogenesis of angiogenesis-related diseases including diabetic retinopathy, retinopathy of prematurity, peripartum cardiomyopathy, and pre-eclampsia [32,33,34]. The influence of the PRL/vasoinhibin axis may extend to RA [6] where excessive angiogenesis helps drive joint inflammation and destruction. PRL stimulates cartilage survival [11, 35] and bone formation [36] and has both proinflammatory and anti-inflammatory effects [6, 37]. We recently reported that PRL protects against joint inflammation, pannus formation, cartilage destruction, and bone loss in adjuvant-induced arthritis [11, 12]. Because angiogenesis is a key event in RA, we reasoned that vasoinhibin, by virtue of its inhibitory effects on blood vessels, could contribute to the protective action of PRL. Here, we show that the intra-articular delivery of the vasoinhibin gene via an AAV2 vector confers protection against joint inflammation, pannus formation, and bone loss in a mouse model of RA.
The AAV2 vasoinhibin vector was able to transduce the vasoinhibin gene in the joint of arthritic and non-arthritic mice without provoking inflammation. Instead, vasoinhibin gene delivery ameliorated the inflammation of arthritic joints as revealed by reduced joint swelling, decreased pannus area and hyperplasia, lowered vascular density, number of vessels, and vasopermeability of the pannus, and reduced mRNA levels of proinflammatory (IL-1β, IL6, ICAM1) and proangiogenic (VEGF, VEGFR2, HIF1α) mediators.
Targeting blood vessels ameliorates arthritis in animal models of RA [4, 38], including bone loss [39, 40]. Our study confirmed the increase in osteoclast number and bone loss reported after AIA induction in mice [41] and the protective effect of angiogenesis inhibitors against bone resorption in arthritis [39, 40]. Therefore, inhibition of blood vessels is the likely cause of the beneficial effect of vasoinhibin on joint inflammation and destruction. Vasoinhibin acts directly on endothelial cells to inhibit the growth, permeability, and dilation of blood vessels [7]. It binds to a multicomponent conformed by plasminogen activator inhibitor 1, urokinase plasminogen activator, and the urokinase plasminogen activator receptor on endothelial cell membranes [42]. These, but also other unidentified binding partners/receptors [43], result in the vasoinhibin blockage of the signaling pathways (Ras-Raf-MAPK; Ras-Tiam1-Rac1-Pak1; PI3K-Akt; and PLCγ-IP3-eNOS) activated by several proangiogenic factors (VEGF, bFGF, bradykinin, IL-1β) [7].
VEGF is central among the various vasoactive substances upregulated in RA and murine arthritis [4, 26, 44]. VEGF dual activity as endothelial cell mitogen and promoter of vascular permeability stimulates the disease by increasing the vascularization and edema that determines pannus formation and joint swelling [26]. Accordingly, blockage of VEGF with sFlt1, the soluble extracellular domain of VEGF receptor 1 [45], or with anti-VEGF antibodies [46, 47], decrease joint inflammation and destruction in collagen-induced arthritis. Here, we show that the injection of VEGF into the knee joint of healthy mice increases local vasopermeability and that co-injection of VEGF with the vasoinhibin protein inhibits this effect. The action of the vasoinhibin protein is similar to that of the AAV2 vasoinhibin vector on arthritis-induced joint vasopermeability, confirming the vascular inhibitory effects of vasoinhibin independent of the delivery method. More importantly, these findings suggest that vasoinhibin can inhibit blood vessel growth and permeability in arthritis by targeting the actions of VEGF. Of note, VEGF can act directly on osteoclasts to promote their bone resorption activity and survival [48]. It remains to be determined whether the beneficial effect of vasoinhibin against bone loss in arthritis may also involve the inhibition of the stimulatory effect of VEGF on osteoclasts and osteoclast precursors.
A main mechanism by which vasoinhibin inhibits VEGF effects on vasoproliferation, vasopermeability, and vasodilation is by blocking the VEGF-induced activation of eNOS [7, 49, 50]. VEGF stimulates eNOS activity via the PI3K/Akt-dependent phosphorylation of eNOS at Ser 1179 [51] and vasoinhibin impairs this action by activating protein phosphatase 2 A that dephosphorylates Ser1179 [30]. Here we show that the phosphorylation of Ser1179 of eNOS is enhanced in the arthritic joint and that the AAV2 vasoinhibin vector inhibits such increase. These findings are consistent with vasoinhibin targeting VEGF-induced eNOS activation in arthritis. However, besides VEGF, there are other vasoactive substances (such as bradykinin, calreticulin) upregulated in the arthritic joint that signal through eNOS that can be targeted by vasoinhibin [7, 52, 53].
A major question is whether disruption of the PRL/vasoinhibin axis influences the progression of RA. The 3 to 1 female to male preponderance of RA and the influence of reproductive states in disease progression have long-held the perception that PRL can trigger or worsen RA [5, 54,55,56]. Basal circulating PRL levels are higher in women than men [57], higher levels of circulating PRL are detected in some patients with RA [58], and inhibition of systemic PRL with the dopamine agonist bromocriptine can be therapeutic [59]. However, the association between PRL levels and disease activity is not consistent. Hyperprolactinemia characterizes pregnancy and lactation when RA goes into remission and exacerbates, respectively [60, 61]; treatment with bromocriptine is not always effective [62]; and dopamine antagonists, causing hyperprolactinemia, can ameliorate the disease [63]. The explanation for these conflicting observations may lie on the ability of PRL to exert immunostimulatory or immunosuppressive effects that depend on complex immune and hormonal interactions [6, 64]. One such interaction may involve the increased production and proteolytic conversion of PRL to vasoinhibin at the inflamed joint. PRL is present in RA synovial fluid [65] and synovium infiltrating T lymphocytes and fibroblast-like synovial cells produce PRL in RA [66]. Furthermore, MMP produced by chondrocytes cleave PRL to vasoinhibin [10], MMP are elevated in the joints of patients with RA [8], and proinflammatory cytokines stimulate MMP production by RA synovial cells [9]. The generation of vasoinhibin, by blocking blood vessel growth and permeability, could be part of the PRL anti-inflammatory effects. However, this proposal is challenged by the known proinflammatory action of MMP production under inflammatory conditions [67] and by evidence showing that vasoinhibin acts as a proinflammatory cytokine in lung tissues [68] where PRL is anti-inflammatory [69, 70]. Further work is needed to understand how PRL and vasoinhibin are mechanistically related and interact to affect vascular and non-vascular components of the inflamed joint.
Finally, our findings add vasoinhibin to the list of promising therapeutics targeting blood vessels in RA. The use of an AAV2 vasoinhibin vector as local gene therapy interfered with joint inflammation by reducing the blood supply needed for the accumulation of immune cells, the proliferation of the inflamed tissue, and the swelling of the joint. AAV2 vectors are an optimal gene therapy strategy for the combined local treatment of isolated joints that are resistant to the systemic approach alone [13]. Further research aimed at tuning the safety and efficacy of the AAV2 vasoinhibin vector to the control of vasoinhibin expression and targeted delivery and action is needed.
References
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Paleolog EM, Miotla JM. Angiogenesis in arthritis: role in disease pathogenesis and as a potential therapeutic target. Angiogenesis. 1998;2:295–307.
Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–39.
Leblond A, Allanore Y, Avouac J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun Rev. 2017;16:594–601.
Neidhart M, Gay RE, Gay S. Prolactin and prolactin-like polypeptides in rheumatoid arthritis. Biomed Pharmacother. 1999;53:218–22.
Clapp C, Adan N, Ledesma-Colunga MG, Solís-Gutiérrez M, Tribiel J, Martínez, de la Escalera G. The role of the prolactin/vasoinhibin axis in rheumatoid arthritis: an integrative overview. Cell Mol Life Sci. 2016;73:2929–48.
Clapp C, Thebault S, Macotela Y, Moreno-Carranza B, Tribiel J, Martínez, de la Escalera G. Regulation of blood vessels by prolactin and vasoinhibins. Adv Exp Med Biol. 2015;846:83–95.
Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Cur Pharm Biotechnol. 2008;9:47–54.
McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42.
Macotela Y, Aguilar MB, Guzmán-Morales J, Rivera JC, Zermeño C, López-Barrera F, et al. Matrix metalloproteases from chondrocytes generate an antiangiogenic 16 kDa prolactin. J Cell Sci. 2006;119:1790–800.
Adan N, Guzman-Morales J, Ledesma-Colunga MG, Perales-Canales SI, Quintanar-Stéphano A, López-Barrera F, et al. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. J Clin Invest. 2013;123:3902–13.
Ledesma-Colunga MG, Adan N, Ortiz G, Solís-Gutiérrez M, López-Barrera F, Martínez de la Escalera G, Clapp C. Prolactin blocks the expression of receptor activator of nuclear factor kappaB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Res Ther. 2017;19:93.
Fabre S, Apparailly F. Gene therapy for rheumatoid arthritis. BioDrugs. 2011;25:381–91.
Coura RdS, Nardi NB. A role for adeno-associated viral vectors in gene therapy. Genet Mol Biol. 2008;31:1–11.
Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J Rheumatol. 2010;37:692–703.
Takahashi H, Kato K, Miyake K, Hirai Y, Yoshino S, Shimada T. Adeno-associated virus vector-mediated anti-angiogenic gene therapy for collagen-induced arthritis in mice. Clin Exp Rheumatol. 2005;23:455–61.
Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–4.
Ramírez M, Wu Z, Moreno-Carranza B, Jeziorski MC, Arnold E, Díaz-Lezama N, Martínez de la Escalera G, et al. Vasoinhibin gene transfer by adenoassociated virus type 2 protects against VEGF- and diabetes-induced retinal vasopermeability. Invest Ophthalmol Vis Sci. 2011;52:8944–50.
Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–9.
van den Berg WB, Joosten LA, van Lent PL. Murine antigen-induced arthritis. Methods Mol Med. 2007;136:243–53.
Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977;20:841–50.
Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–95.
Diaz-Lezama N, Wu Z, Adan-Castro E, Arnold E, Vázquez-Membrillo M, Arredondo-Zamarripa D, et al. Diabetes enhances the efficacy of AAV2 vectors in the retina: therapeutic effect of AAV2 encoding vasoinhibin and soluble VEGF receptor 1. Lab Invest. 2016;96:283–95.
Galfione M, Luo W, Kim J, Hawke D, Kobayashi R, Clapp C, et al. Expression and purification of the angiogenesis inhibitor 16-kDa prolactin fragment from insect cells. Protein Expr Purif. 2003;28:252–8.
Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–30.
Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4:81–90.
Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015;75:3098–107.
Fahey E, Doyle SL. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front Immunol. 2019;10:1426.
Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther. 2008;10:224.
Garcia C, Aranda J, Arnold E, Thébault S, Macotela Y, López-Casillas F, et al. Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J Clin Invest. 2008;118:2291–300.
Triebel J, Bertsch T, Bollheimer C, et al. Principles of the prolactin/vasoinhibin axis. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1193–203.
Triebel J, Macotela Y, Martinez de la Escalera G, Clapp C. Prolactin and vasoinhibins: endogenous players in diabetic retinopathy. IUBMB Life. 2011;63:806–10.
Clapp C, Thebault S, Jeziorski MC, Martinez, de la Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89:1177–215.
Hilfiker-Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014;11:364–70.
Zermeno C, Guzman-Morales J, Macotela Y, Nava G, Lopez-Barrera F, Kouri JB, et al. Prolactin inhibits the apoptosis of chondrocytes induced by serum starvation. J Endocrinol. 2006;189:R1–8.
Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damote D, Goffin V, et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140:96–105.
Costanza M, Binart N, Steinman L, Pedotti R. Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmun Rev. 2015;14:223–30.
Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007;3:434–42.
Chen Y, Donnelly E, Kobayashi H, Debusk LM, Lin PC. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005;52:1585–94.
De Bandt M, Grossin M, Weber AJ, Chopin M, Elbim C, Pla M, et al. Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43:2056–63.
Engdahl C, Lindholm C, Stubelius A, Ohlsson C, Carlsten H, Lagerquist MK. Periarticular bone loss in antigen-induced arthritis. Arthritis Rheum. 2013;65:2857–65.
Bajou K, Herkenne S, Thijssen VL, D’Amico S, Nguyen NQ, Bouche A, et al. PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16K prolactin. Nat Med. 2014;20:741–7.
Clapp C, Weiner R. A specific, high affinity, saturable binding site for the 16-kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology. 1992;130:1380–6.
Paleolog EM. The vasculature in rheumatoid arthritis: cause or consequence? Int J Exp Pathol. 2009;90:249–61.
Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog EM. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest. 2000;80:1195–205.
Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000;164:5922–7.
Sone H, Kawakami Y, Sakauchi M, Nakamura Y, Takahashi A, Shimano H, et al. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem Biophys Res Commun. 2001;281:562–8.
Nakagawa M, Kaneda T, Arakawa T, Morita S, Sato T, Yomada T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000;473:161–4.
Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–9.
Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthal Vis Sci. 1999;40:689–96.
Six I, Kureishi Y, Luo Z, Walsh K. Akt signaling mediates VEGF/VPF vascular permeability in vivo. FEBS Lett. 2002;532:67–69.
Ding H, Hong C, Wang Y, Liu J, Zhang N, Shen C, et al. Calreticulin promotes angiogenesis via activating nitric oxide signalling pathway in rheumatoid arthritis. Clin Exp Immunol. 2014;178:236–44.
Yang A, Zhou J, Wang B, Dai J, Colman RW, Song W, et al. A critical role for plasma kallikrein in the pathogenesis of autoantibody-induced arthritis. FASEB J. 2017;31:5419–31.
Chikanza IC, Grossman AB. Neuroendocrine immune responses to inflammation: the concept of the neuroendocrine immune loop. Baillieres Best Pract Res Clin Rheumatol. 1996;10:199–225.
Brennan P, Ollier B, Worthington J, Hajeer A, Silman A. Are both genetic and reproductive associations with rheumatoid arthritis linked to prolactin? Lancet. 1996;348:106–9.
Hideshi Y, Kazuhiko N, Noboru S. Recent advances in neuro-endocrine-immune interactions in the pathophysiology of rheumatoid arthritis. Curr Rheumatol Rev. 2006;2:191–205.
Beltran L, Fahie-Wilson MN, McKenna TJ, Kavanagh L, Smith TP. Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: evaluation and validation on common immunoassay platforms. Clin Chem. 2008;54:1673–81.
Orbach H, Zandman-Goddard G, Amital H, Barak V, Szekanecz Z, Szucs G, et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann NY Acad Sci. 2007;1109:385–400.
McMurray RW. Bromocriptine in rheumatic and autoimmune diseases. Semin Arthritis Rheum. 2001;31:21–32.
Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29:185–91.
Barrett JH, Brennan P, Fiddler M, Silman A. Breast‐feeding and postpartum relapse in women with rheumatoid and inflammatory arthritis. Arthritis Rheum. 2000;43:1010–5.
Salesi M, Sadeghihaddadzavareh S, Nasri P, Namdarigharaghani N, Farajzadegan Z, Hajalikhani M. The role of bromocriptine in the treatment of patients with active rheumatoid arthritis. Int J Rheum Dis. 2013;16:662–6.
Grimaldi MG. Long-term low dose haloperidol treatment in rheumatoid patients: effects on serum sulphydryl levels, technetium index, ESR, and clinical response. Br J Clin Pharmacol. 1981;12:579–81.
Yu-Lee LY. Prolactin modulation of immune and inflammatory responses. Recent Prog Horm Res. 2002;57:435–55.
Ogueta S, Munoz J, Obregon E, Delgado-Baeza E, García-Ruiz JP. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol Cell Endocrinol. 2002;190:51–63.
Nagafuchi H, Suzuki N, Kaneko A, Asai T, Sakane T. Prolactin locally produced by synovium infiltrating T lymphocytes induces excessive synovial cell functions in patients with rheumatoid arthritis. J Rheumatol. 1999;26:1890–900.
Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4:128–35.
Corbacho AM, Nava G, Eiserich JP, Noris G, Macotela Y, Struman I. Proteolytic cleavage confers nitric oxide synthase inducing activity upon prolactin. J Biol Chem. 2000;275:13183–6.
Corbacho AM, Macotela Y, Nava G, Eiserich JP, Cross CE, Martínez de la Escalera G, et al. Cytokine induction of prolactin receptors mediates prolactin inhibition of nitric oxide synthesis in pulmonary fibroblasts. FEBS Lett. 2003;544:171–5.
Ochoa-Amaya JE, Hamasato EK, Tobaruela CN, Queiroz-Hazarbassanov N, Anselmo Franci JA, Palermo-Neto J, et al. Short-term hyperprolactinemia decreases allergic inflammatory response of the lungs. Life Sci. 2015;142:66–75.
Acknowledgements
The authors thank Fernando López Barrera, Xarubet Ruiz Herrera, Alejandra Castilla, Martín García, Maarten Werdler, Ericka A. de los Ríos, Michael C. Jeziorski, Daniel Mondragón, and Antonio Prado for their excellent technical assistance. Georgina Ortiz is a doctoral student from the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 278266 from the National Council of Science and Technology of Mexico (CONACYT). The work was supported by UNAM grant PAPIIT IN200518 to CC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ortiz, G., Ledesma-Colunga, M.G., Wu, Z. et al. Vasoinhibin reduces joint inflammation, bone loss, and the angiogenesis and vasopermeability of the pannus in murine antigen-induced arthritis. Lab Invest 100, 1068–1079 (2020). https://doi.org/10.1038/s41374-020-0432-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-0432-5