Abstract
Sparc/osteonectin, cwcv, and kazal-like domain proteoglycan 1 (SPOCK1) is a matricellular protein which regulates cell proliferation, invasion, and survival but the function of SPOCK1 in liver fibrosis is obscure. In this study, we found that SPOCK1 expression increased significantly in fibrotic liver tissues and activated primary rat hepatic stellate cells (R-HSCs). SPOCK1 co-localized with α-smooth muscle actin (α-SMA) in the cytoplasm. Mechanistically, we found platelet-derived growth factor-BB (PDGF-BB) induced SPOCK1 expression by activating the PI3K/Akt/forkhead box M1 (FoxM1) signaling pathway. Intracellular SPOCK1 downregulation decreased the HSC activation, proliferation, and migration induced by PDGF-BB. Furthermore, intracellular SPOCK1 overexpression or recombinant SPOCK1 treatment promoted HSC activation, proliferation, and migration by activating the PI3K/Akt signaling pathway. Co-immunoprecipitation, double immunofluorescence staining indicated that SPOCK1 interacted with integrin α5β1, and neutralization of integrin α5β1 significantly reduced the role of recombinant SPOCK1 in HSCs. In vivo HSC-specific SPOCK1 knockdown following lentivirus administration dramatically ameliorated thioacetamide (TAA)-induced collagen deposition in rat livers. Collectively, our study indicates that SPOCK1 is crucial for hepatic fibrosis and it might be a promising therapeutic target.
Similar content being viewed by others
Introduction
Liver fibrosis is wound-healing response characterized by excessive extracellular matrix (ECM) deposition [1]. Globally, liver cirrhosis nearly leads to 1.16 million deaths each year [2], however, treatment remains limited [3]. Hepatic stellate cell (HSC) activation is a key event for liver fibrogenesis [4], and this process is controlled by multiple soluble mediators, especially transforming growth factor (TGF)-β1, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), and vascular endothelial growth factor (VEGF) [5]. PDGF is a family of homodimers or heterodimers, among which PDGF-BB exerts the strongest pro-fibrogenic effect [6].
Matricellular proteins are a family of soluble cytokines and matrix-bound proteins that can be intracellular or secreted [7]. Rather than exert a primary role in tissue architecture, these nonstructural ECM proteins are typically restricted to embryonic development, inflammation, tissue remodeling, and tumor progression [8]. Sparc/osteonectin, cwcv, and kazal-like domain proteoglycan 1 (SPOCK1), initially identified in the human testis, is a member of the secreted protein, acidic, and rich in cysteine (SPARC) family [9]. The SPOCK1 protein is encoded by SPOCK1 gene, and although its function is unknown, some studies suggest that its function may be related to protease inhibition [10, 11]. Several studies have demonstrated that SPOCK1 regulates cell proliferation, invasion, survival, and promotes the progression of several cancers, including hepatocellular carcinoma, esophageal squamous cell carcinoma, colorectal cancer, and gallbladder cancer [12,13,14,15]. In addition, SPOCK1 is a confirmed TGF-β1 target gene in breast cancer cells and lung cancer cells [16, 17]. Interestingly, SPARC contributes to pro-fibrogenic HSC responses and liver fibrosis [18, 19]. However, to our knowledge, the function of SPOCK1 in liver fibrosis has never been reported, nor has the underlying mechanism.
In this study, we report the pro-fibrogenic effect of SPOCK1 in hepatic fibrosis. SPOCK1 is a downstream molecule of PDGF-BB and it promotes HSC activation, proliferation, and migration by activating the integrin α5β1/PI3K/Akt signaling pathway, thus promoting liver fibrosis. In addition, HSC-specific inhibition of SPOCK1 dramatically ameliorates thioacetamide (TAA)-induced rat liver fibrosis. This study may therefore identify a potential interventional target for liver fibrosis.
Materials and methods
Reagents
Recombinant TGF-β1, PDGF-BB, CTGF, and VEGF were from Peprotech (Rocky Hill, CT, USA). Recombinant SPOCK1 and neutralizing antibodies against human integrin α5, integrin β1 were from R&D systems (Minnesota, USA). Neutralizing antibodies against rat integrin α5, integrin β1 were from BioLegend (San Diego, USA). PI3K inhibitor LY294002 (HY-10108) and ERK inhibitor U0126 (HY-12031) were from MedChem Express (New Jersey, USA). Detailed information of the reagents was listed in Supplementary Table S1. All the reagents were used according to the manufacturers’ instructions.
Human liver samples
In total, 50 liver samples were acquired from patients who received surgery at Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology (HUST), including 10 samples from liver hemangioma patients and 40 samples from liver cancer patients (the adjacent liver tissues were confirmed to be cirrhotic by pathologists). The study acquired approval from the Ethics Committee of Tongji Hospital, and the study was conducted following the principles of the Declaration of Helsinki. A solution of 4% paraformaldehyde was used for fixation and paraffin was applied for embedding, the samples were used for immunohistochemical (IHC) or immunofluorescence (IF) staining.
Rat fibrotic models and HSC-specific gene knockdown
Rat fibrotic models: we randomly divided male Sprague Dawley (SD) rats (about 250 g) into a normal group and a fibrotic group (ten rats per group). In the fibrotic group, TAA (dissolved using saline, 150 mg/kg body weight) was injected intraperitoneally into rats two times weekly for 8 weeks to induce chronic hepatic fibrotic models. Accordingly, an equal volume of PBS was injected into the rats in the normal group.
HSC-specific gene knockdown: we randomly divided 40 male SD rats (about 250 g) into four groups (n = 10). To make a HSC-specific gene delivery for SPOCK1 knockdown, a lentiviral construct comprising the sequences targeted at SPOCK1 (targeted sites: 5′-GCAGTGCTGGTGCGTGGATAA-3′) was cloned downstream of the rat α-smooth muscle actin (a-SMA) gene promoter (Genechem, Shanghai, China), designated as LV-SMA-shSPOCK1-Flag. The structure of the lentivirus is similar to the construct used by Koo et al., which can specifically target HSCs [20]. For HSC-specific gene delivery experiments in vivo, an aliquot of 100 µl 5.0 × 107 TU was administered via tail vein to each rat in group IV, accordingly, equivalent amount of the control lentivirus (designated as LV-MOCK) was administered to rats in group III. After 7 days, the rats in group II (TAA group), III (TAA + LV-MOCK group), and IV (TAA + LV-SMA-shSPOCK1-Flag group) were injected with TAA (150 mg/kg body weight) two times weekly for 6 weeks. As a comparison, the equal volume of PBS was injected into rats of group I. Forty-eight hours after the last injection, rats were killed and the tissues used for analysis. All the animal studies were performed following the national and international guidelines, and the study acquired the approvement from the Ethics Committee of Animal Experiments of Tongji Medical College and supervised by the Department of Experimental Animals of the Tongji Medical College, HUST.
Cell culture
SD rats were used for isolation of primary rat HSCs (R-HSCs) as described previously [21]. The R-HSCs were then cultivated in DMEM containing 20% FBS [22]. LX-2 (a human HSC cell line) were acquired from the Institute of Liver and Gastrointestinal Diseases, HUST and cultivated in DMEM containing 10% FBS.
IHC and IF staining
The detailed protocols for IHC and IF staining were described previously [22]. The primary antibodies used in the IHC assay included SPOCK1, α-SMA, Flag, and collagen A1 (COL1A1). We employed negative controls for staining, and in the negative control group, the primary antibodies were replaced by PBS. The severity of hepatic fibrosis was assessed by Masson’s trichrome staining. For IF staining, the primary antibodies included α-SMA, SPOCK1, integrin β1, Desmin, and Flag. Digital photos were obtained using a confocal microscopy or a fluorescence microscope (Olympus). The antibodies are listed in Supplementary Table S1.
Plasmid construction
Standard procedures were used for plasmid construction as previously described [23]. Relevant primers are listed in Supplementary Table S2. The (−1781/+48) SPOCK1 promoter construct was produced using human genomic DNA. The construct represented the sequence from −1781 to +48 (versus the transcriptional start site (TSS)) of the 5′-flanking region of the human SPOCK1 gene. The construct was produced using the primers which incorporated KpnI and MluI sites at the 5′ and 3′-ends, respectively. The PCR product was then cloned into the KpnI and MluI sites of the pGL3-Basic vector (Promega, USA). The 5′-flanking deletion constructs of the SPOCK1 promoter, (−1356/+48) SPOCK1, (−741/+48) SPOCK1, (−237/+48) SPOCK1, (−50/+48) SPOCK1, were produced, and the (−1781/+48) SPOCK1 construct was applied as template. The QuikChange II Site-Directed Mutagenesis Kit (Stratagene, CA, USA) was applied for site-directed mutation in the SPOCK1 promoter. DNA sequencing was conducted to verify the constructs.
Construction of lentivirus and stable cell lines
Lentiviral vectors containing shRNAs were purchased from Genechem (Shanghai, China), separately designated as human LV-shSPOCK1 (targeted sites: 5′-GCTTTCGAGACGATGATTATT-3′) and rat LV-shSPOCK1 (targeted sites: 5′-GCAGTGCTGGTGCGTGGATAA-3′), “LV-sh control” is a nontarget shRNA. Lentiviral vectors encoding the human and rat SPOCK1 genes were purchased from Genechem and designated as human and rat LV-SPOCK1. The “LV-control” is an empty vector. The multiplicity of infection for lentivirus transfection is 100, in the presence of polybrene. Seventy-two hours after infection, puromycin (OriGene) was applied for 2 weeks for HSC cells selection. After confirming the transfection efficiency, the stable knockdown and overexpressing HSC cells were used for the further experiments.
SiRNA, plasmid transient transfection
The human FoxM1 siRNA and relevant control siRNA were from Riobio (Guangzhou, China). Human SPOCK1 plasmid was from Genechem. The siRNA or plasmid transfection was performed following the standard protocols using Lipofectamine 3000 (Invitrogen). The real-time PCR and western blotting analyses were conducted to verify the transfection efficiency. The sequences of siRNA are presented in Supplementary Table S2.
Luciferase reporter assay
A dual luciferase assay (Promega) was conducted to detect the luciferase activity following standard protocols described previously [23]. The transfected cells were lysed, subsequently, the lysates were centrifuged at the maximum velocity for 1 min. Relative luciferase activity was detected applying a ModulusTM TD20/20 Luminometer (Turner Biosystems, USA), and the Renilla activity was used as the control for the normalization of transfection efficiencies.
RNA extraction and quantitative real-time PCR (qRT-PCR)
The standard procedures for RNA extraction and qRT-PCR were described previously [22]. The primer sequences are presented in Supplementary Table S2.
Western blotting and co-immunoprecipitations (co-IP)
The standard protocol for western blotting assay was described previously [22]. Protein was extracted from liver tissue, R-HSCs, and LX-2 cells. After electrophoresis, the membranes were treated with specific antibodies overnight at 4 °C, and then incubated with secondary antibodies. An ECL assay kit (Peptbio, Wuhan, China) was used to detect the blots. For the co-IP assay, 1% NP-40 buffer (Promoter) was applied for cell lysis, and the supernatants were incubated with protein G-Sepharose beads conjugated with mouse anti-SPOCK1 or anti-IgG antibody overnight at 4 °C. Subsequently, western blotting assay was performed to detect the samples. Detailed antibodies information is presented in Supplementary Table S1.
Chromatin immunoprecipitation analysis (ChIP)
The standard procedures for ChIP analysis were described previously [24]. Briefly, the LX-2 were soaked in 1% formaldehyde for cross-linking and quenched using glycine before harvest and sonication. Then, the sheared chromatin got incubation with primary antibodies against FoxM1 and isotype control IgG for 2 h, together with protein G-Sepharose beads and herring sperm DNA. After retrieval from beads, the immunoprecipitated DNA was purified. Subsequently, qRT-PCR analysis was utilized to quantify the corresponding binding site on the promoter. The primer sequences are shown in Supplementary Table S2.
Cell counting kit-8 (CCK-8) analysis
CCK-8 analysis was performed to detect HSC proliferation capability, and the detailed procedures were described previously [22].
Transwell analysis
Transwell analysis was conducted to assess HSC migratory ability, following the previous protocol [22]. Briefly, 2 × 104 cells were resuspended in 200 μl DMEM (serum-free), and then plated in the upper chamber (8 μm, Corning, USA), 600 μl DMEM containing 20% FBS (or PDGF-BB (20 ng/ml) or rSPOCK1 (100 ng/ml)) was added to the bottom chamber. After 48 h, cells which had migrated to the lower layers got fixed with methanol and stained using crystal violet, and then photos were taken using a microscope and numbers were recorded. Five fields of each insert were counted, the means were calculated, and this analysis was conducted thrice.
Hydroxyproline assay
Hydroxyproline content was evaluated in liver samples obtained at the end of the in vivo experiment and determined by a clinical test kit (Jiancheng, Nanjing, China) according to the standard protocol.
Quantification and statistical analysis
SPSS software (version 19.0) was adopted for statistical analysis. Data from at least three independent experiments were presented as the mean ± SD. Student’s t test and ANOVA were adopted to analyze the data. Pearson’s rank correlation test was applied to determine the correlation coefficients. When P < 0.05, the statistical significance was confirmed.
Results
SPOCK1 expression is upregulated in liver fibrosis and HSC activation
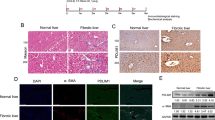
Human normal liver tissues and cirrhotic liver tissues were detected. IHC analysis revealed that the cirrhotic liver tissues had larger fibrotic areas, as shown by Masson’s trichrome staining, higher α-SMA and higher SPOCK1 expression than those of normal liver tissues (Fig. 1a). Furthermore, α-SMA and SPOCK1 levels were significantly higher in cirrhotic tissues (Fig. 1b, c). In the analysis of a human cirrhotic liver cohort database (GSE25097), SPOCK1 mRNA level was positively correlated with α-SMA in cirrhotic liver (Fig. 1d). These results indicate that SPOCK1 is upregulated in the liver of cirrhosis patients, suggesting that SPOCK1 might play a role in HSC activation.
a Representative images of immunohistochemical (IHC) analysis for α-SMA and SPOCK1 and Masson’s trichrome staining in human normal and cirrhotic liver tissues. Scale bars: 100 μm. b The mRNA levels of α-SMA and SPOCK1 in normal (n = 10) and cirrhotic human liver tissues (n = 40) was evaluated by RT-qPCR analysis. c Analysis of α-SMA and SPOCK1 protein levels in normal liver tissues (n = 3) and cirrhotic human liver tissues (n = 5) by western blotting analysis. d Pearson’s correlation analysis of α-SMA and SPOCK1 mRNA levels in a cohort of fibrosis patients (GSE25097) (n = 46). e Representative images of IHC analysis for α-SMA and SPOCK1 and Masson’s trichrome staining in rat normal and TAA-induced fibrotic liver tissues. Scale bars: 100 μm. f The α-SMA and SPOCK1 levels in quiescent (1 day) and activated (10 day) R-HSCs were detected using RT-qPCR analysis and western blotting analysis. g SPOCK1 and α-SMA were co-stained in human cirrhotic liver tissues by immunofluorescence. Scale bars: 50 μm. h SPOCK1 and α-SMA were co-stained in activated R-HSCs by immunofluorescence. Scale bars: 20 μm. ***P < 0.001 vs. control.
Next, we analyzed SPOCK1 expression in normal and TAA-induced fibrotic rats liver tissues. IHC analysis also revealed larger fibrotic areas and higher α-SMA and SPOCK1 expression in fibrotic group (Fig. 1e). Then, we isolated and cultured R-HSCs, which were quiescent on the first day and activated on the tenth day. SPOCK1 expression increased significantly following R-HSC activation (Fig. 1f).
Furthermore, double IF staining of SPOCK1 and α-SMA was performed using the human cirrhotic liver tissues and activated R-HSCs. The results show that SPOCK1 co-localized with α-SMA both in liver tissue and R-HSCs, indicating that SPOCK1 was localized in the cytoplasm of HSCs (Fig. 1g, h). These data demonstrate that SPOCK1 is upregulated in liver fibrosis and HSC activation and might be involved in the pathogenesis of hepatic fibrogenesis.
PDGF-BB upregulates expression of SPOCK1 by activating the PI3K/Akt/FoxM1 signaling pathway
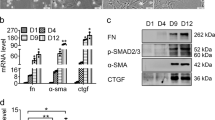
To determine the potential upstream regulatory factors of SPOCK1, LX-2 cells, and R-HSCs were treated with equivalent concentration of recombinant TGF-β1, PDGF-BB, CTGF, and VEGF. TGF-β1 and PDGF-BB significantly increased SPOCK1 expression, and PDGF-BB exerted a stronger effect, while the effect of CTGF and VEGF was insignificant (Fig. 2a). Next, we examined whether PDGF-BB promoted SPOCK1 expression by transactivating its promoter. LX-2 cells were subjected to PDGF-BB after transfecting a reporter plasmid containing the SPOCK1 gene ((−1781/+48) SPOCK1) promoter. The results of luciferase reporter assay demonstrated that the SPOCK1 promoter activity increased dramatically after PDGF-BB treatment (Fig. 2b, left).
a LX-2 cells and R-HSCs were exposed to equivalent concentration (20 ng/ml) of recombinant TGF-β1, PDGF-BB, CTGF, and VEGF for 48 h (DMSO as control), followed by RT-qPCR and western blotting analyses to detect SPOCK1 expression. b (Left) Relative luciferase activity was evaluated in LX-2 cells after transfection of the reporter plasmid containing the SPOCK1 promoter and incubated with PDGF-BB (20 ng/ml) for 48 h or not. (Right) Serially truncated and mutated SPOCK1 promoter constructs were cloned into pGL3-luciferase reporter plasmids. After plasmids transfection, LX-2 were subjected to PDGF-BB, then the luciferase activity was detected. c After FoxM1 siRNA transfection, LX-2 were subjected to PDGF-BB (20 ng/ml) or not. Forty-eight hours after PDGF-BB treatment, the promoter activity and level of SPOCK1 were assessed. d After LY294002 or U0126 (25 μM) pretreatment for 2 h, LX-2 were subjected to PDGF-BB (20 ng/ml) for 48 h or not. The levels of SPOCK1, phosphorylation, and expression of Akt and ERK were evaluated. e Direct binding between FoxM1 and SPOCK1 promoter was assessed by ChIP analysis, which was induced by PDGF-BB through activation of the PI3K/Akt signaling pathway. ***P < 0.001; **P < 0.01; #, not significant vs. control.
To clarify the exact location of potential transcription factors on the SPOCK1 promoter that respond to PDGF-BB regulation, the truncated or mutant SPOCK1 promoter sequences were generated into reporter plasmid constructs. These plasmids were then transfected into LX-2 cells to evaluate their response to PDGF-BB treatment. The data showed deletion from nt-1356 to nt-741 dramatically reduced PDGF-BB-enhanced SPOCK1 promoter activity, suggesting that the sequence was critical for PDGF-BB-induced SPOCK1 promoter activity. Notably, this region contained three forkhead box M1 (FoxM1) putative binding sites. Interestingly, mutation of FoxM1 putative binding site 1 significantly attenuated PDGF-BB-induced SPOCK1 promoter activity, whereas mutation of FoxM1 putative binding site 2 or 3 had no significant effect (Fig. 2b, right). In addition, a luciferase reporter assay demonstrated that FoxM1 knockdown obviously suppressed PDGF-BB-mediated SPOCK1 promoter transactivation. RT-qPCR and western blotting analyses also showed that FoxM1 knockdown significantly decreased PDGF-BB-induced SPOCK1 expression (Fig. 2c).
Mechanistically, PDGF-BB activates the canonical downstream effectors Akt and ERK, and subsequently mediates transcription of pro-fibrogenic genes [25]. To elucidate which pathway is involved in PDGF-BB-mediated SPOCK1 expression, LX-2 were incubated with PI3K inhibitor (LY294002) and ERK inhibitor (U0126) before subjected to PDGF-BB treatment. Pretreatment of cells with LY294002 reduced PDGF-BB-induced SPOCK1 expression. However, U0126 pretreatment exerted no obvious effect (Fig. 2d). Furthermore, the ChIP analysis results showed that LY294002 notably decreased the binding of FoxM1 to SPOCK1 promoter, while U0126 exerted no obvious effect (Fig. 2e). Collectively, the results indicate that PDGF-BB upregulates the expression of SPOCK1 in HSCs by activating the PI3K/Akt/FoxM1 signaling pathway.
Intracellular SPOCK1 mediates PDGF-BB-induced HSC activation, proliferation, and migration
PDGF-BB is a strong promotor of HSC activation, proliferation, and migration [6, 26, 27]. To determine whether intracellular SPOCK1 mediates PDGF-BB-induced pro-fibrogenic responses, we stably downregulated SPOCK1 expression in LX-2 cells and R-HSCs by lentiviral transfection, the corresponding cells were designated as “sh control” and “shSPOCK1” cells; then, the indicated cells were subjected to PDGF-BB. The results showed that PDGF-BB significantly enhanced α-SMA and COL1A1 levels, on the contrary, SPOCK1 downregulation attenuated PDGF-BB-induced HSC activation (Fig. 3a). In addition, CCK-8 and Transwell analyses separately showed that PDGF-BB significantly enhanced HSC proliferation and migration, which were decreased due to SPOCK1 knockdown (Fig. 3b, c).
a LX-2 cells and R-HSCs were separately transfected with SPOCK1-knockdown lentivirus (shSPOCK1) or the control lentivirus (sh control). Then, the stably transfected cells were subjected to PDGF-BB (20 ng/ml) or not for 48 h. HSC activation markers (α-SMA and COL1A1) were measured by RT-qPCR and western blotting analyses. b Proliferation was assessed by CCK-8 analysis. c Migration was measured by Transwell analysis. Scale bars: 100 μm. d RT-qPCR and western blotting analyses were conducted to detect CyclinB1, CyclinD1, CyclinE1, MMP2, and MMP9 levels in LX-2 cells. ***P < 0.001; **P < 0.01; *P < 0.05; #, not significant vs. control.
Furthermore, knockdown of SPOCK1 significantly decreased PDGF-BB-enhanced expression of CyclinB1, CyclinD1, matrix metalloproteinase (MMP) 2 and MMP9 in LX-2 cells (Fig. 3d), and R-HSCs (Supplementary Fig. S1a) without affecting the expression of CyclinE1. These results suggest that intracellular SPOCK1 mediates PDGF-BB-induced HSC activation, proliferation, and migration.
Intracellular SPOCK1 regulates HSC activation, proliferation, and migration by activating the PI3K/Akt signaling pathway
Next, we investigated whether intracellular SPOCK1 itself regulated the pro-fibrogenic response of HSCs. We stably upregulated SPOCK1 expression in LX-2 cells and R-HSCs by lentiviral infection, and the corresponding cells were designated as “control” and “SPOCK1” cells. To determine which signaling pathway is regulated by altering SPOCK1 expression, the phosphorylated level and total expression of Akt, ERK, JNK, and p38 were assessed, which were reported to regulate HSCs biological function [22]. The results showed that SPOCK1 knockdown decreased Akt phosphorylation, while SPOCK1 overexpression increased Akt phosphorylation, without affecting the expression of total Akt. However, altering SPOCK1 expression did not affect the levels of phosphorylated and total ERK, JNK, and p38 (Fig. 4a). These data suggest that intracellular SPOCK1 activates the PI3K/Akt signaling pathway.
SPOCK1-knockdown lentivirus (shSPOCK1) or the control lentivirus (sh control), and SPOCK1-overexpressing lentivirus (SPOCK1) or the control lentivirus (control) were separately transfected into LX-2 cells. Puromycin used to select the stable transfected cells for 2 weeks. Then, the indicated cells were plated in the six-well plates. After 48 h, the protein was extracted. The SPOCK1 expression and phosphorylation and expression of Akt, ERK, JNK, and p38 were determined. a The indicated lentivirus stable transfected HSC cells were plated in six-well plate on the first day. On the second day, the cells were subjected to LY294002 (25 μM) for 2 h. After culturing for another 48 h, the mRNA and protein levels of α-SMA and COL1A1 were detected. b Proliferation was assessed by CCK-8 analysis. c, d Migration was measured by Transwell analysis. e The indicated lentivirus stable transfected LX-2 were plated on the first day. On the second day, the cells were subjected to LY294002 (25 μM) for 2 h. After culturing for another 48 h, the expression of CyclinB1, CyclinD1, MMP2, and MMP9 in indicated LX-2 cells was assessed by RT-qPCR and western blotting analyses. ***P < 0.001, **P < 0.01, *P < 0.05 vs. control.
Next, further studies were conducted to investigate whether SPOCK1 can regulate pro-fibrogenic HSCs responses by activating the PI3K/Akt signaling pathway. The LX-2 and R-HSCs with stable overexpression of SPOCK1 and the control cells were administered with LY294002, and HSC activation, proliferation, and migration were analyzed. SPOCK1 overexpression dramatically upregulated α-SMA and COL1A1 expression, and enhanced proliferation and migration capability of HSCs, however, treatment with LY294002 significantly decreased the effect of SPOCK1 overexpression (Fig. 4b–d).
Moreover, inhibition of the PI3K/Akt signaling pathway significantly decreased intracellular SPOCK1-induced CyclinB1, cyclinD1, MMP2, and MMP9 expression in HSCs (Fig. 4e and Supplementary Fig. S1b). These data suggest that intracellular SPOCK1 regulates HSC activation, proliferation, and migration by activating PI3K/Akt signaling.
Recombinant SPOCK1 (rSPOCK1) regulates HSC activation, proliferation, and migration by activating PI3K/Akt signaling
As a matricellular protein, SPOCK1 can remain intracellularly or be secreted into the ECM. Thus, we utilized rSPOCK1 to further evaluate the role of SPOCK1 in HSCs. LX-2 cells were subjected to rSPOCK1, and the phosphorylation and expression of Akt, ERK, JNK, and p38 were analyzed using western blotting. These results show that rSPOCK1 treatment promoted Akt phosphorylation without affecting the expression of total Akt. However, rSPOCK1 did not affect the levels of phosphorylated and total MAPKs (Supplementary Fig. S2a). These data suggest that extracellular SPOCK1 treatment also activates the PI3K/Akt signaling pathway.
To investigate whether rSPOCK1 exerted its effect by activating the PI3K/Akt signaling pathway, HSCs were pretreated with LY294002 and then treated with rSPOCK1, subsequently, HSC activation, proliferation, and migration were analyzed. Similarly, LY294002 treatment dramatically abolished rSPOCK1-enhanced expression of α-SMA and COL1A1, proliferation, and migration of HSCs (Supplementary Fig. S2b–d).
Moreover, LY294002 treatment significantly decreased rSPOCK1-induced cyclinB1, cyclinD1, MMP2 and MMP9 expression in LX-2 cells (Supplementary Fig. S2e), and R-HSCs (Supplementary Fig. S1c). These data suggest that rSPOCK1 regulates HSC activation, proliferation, and migration by activating the PI3K/Akt signaling pathway.
SPOCK1 interacts with integrin α5β1 and upregulates HSC activation, proliferation, and migration by activating the PI3K/Akt signaling pathway
Several matricellular proteins, such as osteopontin and periostin, have been reported to promote HSCs collagen synthesis, proliferation, and migration by interacting with various integrin subtypes [28, 29]. However, it has never been reported whether SPOCK1 interacts with integrins. To date, the integrins that are expressed in HSCs include integrin α1β1, α2β1, α5β1, α6β4, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, αVβ8, α8β1, and α11β1 [30, 31]. To determine whether SPOCK1 interacts with integrins, co-IP was performed to analyze the immunoprecipitates from Flag-tagged SPOCK1-transfected LX-2 cells. The data reveal that SPOCK1 interacted with integrin α5β1 (Fig. 5a). In addition, the interaction between SPOCK1 and integrin β1 was confirmed by double IF staining in LX-2 cells (Fig. 5b). Next, specific neutralizing antibodies against integrin α5 (anti-α5) or integrin β1 (anti-β1) were used to determine whether blocking integrin α5 or integrin β1 attenuated rSPOCK1-induced HSCs fibrogenic responses. The data demonstrate that anti-α5 and anti-β1 dramatically abolished rSPOCK1-induced α-SMA and COL1A1 expression, proliferation, and migration of HSCs, and a combination of anti-α5 and anti-β1 exerted a synergistic inhibitory effect (Fig. 5c–e and Supplementary Fig. S3a–c).
After Flag-tagged SPOCK1 plasmid transfection, LX-2 lysates were immunoprecipitated using SPOCK1 antibody and isotype normal IgG antibody. Western blotting analysis was performed to detect integrin −α1, −α2, −α5, −α6, −α8, −α11, −αv, −β1, −β3, −β4, −β5, −β6, and −β8 and SPOCK1 in this immunoprecipitation. a Double immunofluorescence staining of SPOCK1 and integrin β1 in LX-2 cells. Scale bars: 20 μm. b LX-2 cells were subjected to rSPOCK1 (100 ng/ml) for 48 h with specific neutralizing antibodies against integrin α5 (5 μg/ml), integrin β1 (5 μg/ml), or both, subsequently, α-SMA and COL1A1 levels were analyzed. d CCK-8 analysis of LX-2 cells after treatment with rSPOCK1 (100 ng/ml) with or without neutralizing antibodies against integrin α5 (5 μg/ml), integrin β1 (5 μg/ml), or both. e Transwell analysis of LX-2 cells towards rSPOCK1 (100 ng/ml) with or without neutralizing antibodies against integrin α5 (5 μg/ml), integrin β1 (5 μg/ml), or both. f LX-2 cells were subjected to rSPOCK1 (100 ng/ml) with or without neutralizing antibodies against integrin α5 (5 μg/ml), integrin β1 (5 μg/ml), or both. Forty-eight hours later, the expression of CyclinB1, CyclinD1, MMP2, MMP9, p-Akt, and Akt in LX-2 cells were analyzed. ***P < 0.001, **P < 0.01, *P < 0.05 vs. control.
Moreover, blocking integrin α5 or integrin β1 significantly decreased rSPOCK1-induced CyclinB1, CyclinD1, MMP2 and MMP9 expression, and the phosphorylation of Akt (Fig. 5f and Supplementary Fig. S3d). These data suggest that SPOCK1 interacts with integrin α5β1, activates the intracellular PI3K/Akt signaling pathway, and subsequently promotes HSC activation, proliferation, and migration.
HSC-specific SPOCK1 knockdown alleviates TAA-induced rat liver fibrosis
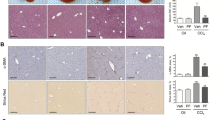
To investigate the effect of HSC SPOCK1 in liver fibrosis, we made a lentiviral SMA-shSPOCK1-Flag (LV-SMA-shSPOCK1-Flag) delivery system (a construct composed of the sequences targeting SPOCK1 downstream of the α-SMA promoter) and injected the packaged lentivirus into rats via the tail vein to accomplish HSC-specific SPOCK1 knockdown (Fig. 6a). The rats were sacrificed for analysis 6 weeks after TAA or PBS injection. The successful delivery of LV-SMA-shSPOCK1-Flag to HSCs was evaluated by selective Flag expression in the perisinusoidal area and by Flag co-staining with desmin-positive cells (Fig. 6a, left and middle). Furthermore, HSC-specific delivery was verified by using isolated R-HSCs, and western blotting analysis showed that Flag was only expressed in R-HSCs from rats that had been injected with LV-SMA-shSPOCK1-Flag rather than with LV-MOCK (Fig. 6a, right). Subsequently, IHC staining showed that TAA administration dramatically increased collagen deposition (represented by Masson’s trichrome staining), α-SMA and COL1A1 expression, which were significantly ameliorated as a consequence of HSC-specific SPOCK1 inhibition (Fig. 6b). In addition, LV-SMA-shSPOCK1-Flag administration significantly reduced the hydroxyproline content in the liver, which increased significantly after TAA administration (Fig. 6c). Moreover, HSC-specific SPOCK1 knockdown significantly decreased the serum ALT and AST level in rats which were enhanced by TAA treatment (Supplementary Fig. S4). Furthermore, α-SMA, COL1A1, CyclinB1, CyclinD1, MMP2, and MMP9 expression decreased dramatically in LV-SMA-shSPOCK1-Flag-administered rats compared with those in the LV-MOCK-administered rats, both in the liver tissues and in freshly isolated R-HSCs (Fig. 6d, e). Taken together, these results indicate that HSC-specific SPOCK1 inhibition ameliorates TAA-induced liver fibrosis.
a Lentiviral SMA-shSPOCK1-Flag delivery to HSCs in rats. (Left panel) Liver sections were immunostained for Flag. Arrows indicate Flag-positive cells. Scale bars: 50 and 20 μm. (Middle panel) Double immunofluorescence staining of Flag and desmin. Arrows indicate desmin-positive cells. Scale bars: 20 μm. (Right panel) R-HSCs were isolated at the end of the in vivo experiment, and the expression of Flag was analyzed by western blotting. b Representative images of IHC analysis of Masson’s trichrome, α-SMA, COL1A1 (Scale bars: 100 μm) in the four groups (n = 6). c Liver hydroxyproline content in the four groups (n = 6) was assessed. d Expression of α-SMA, COL1A1, CyclinB1, CyclinD1, MMP2, MMP9, and SPOCK1 in the liver of LV-MOCK and LV-SMA-shSPOCK1-Flag -administered rats were measured by RT-qPCR (n = 6) and western blotting (n = 4) analyses. e Expression of α-SMA, COL1A1, CyclinB1, CyclinD1, MMP2, MMP9, and SPOCK1 in freshly isolated R-HSCs from LV-MOCK and LV-SMA-shSPOCK1-Flag-administered rats were measured by RT-qPCR and western blotting analyses. f Schematic representation of a working model by which SPOCK1 promotes liver fibrosis. The expression of SPOCK1 is upregulated by PDGF-BB by activating the PI3K/Akt/FoxM1 signaling pathway. SPOCK1 interacts with integrin α5β1 to activate the PI3K/Akt signaling pathway to promote HSC activation, proliferation, and migration. HSC-specific SPOCK1 knockdown ameliorates TAA-induced liver fibrosis. ***P < 0.001, **P < 0.01, *P < 0.05 vs. control.
Discussion
Liver fibrosis is generally orchestrated by five essential components: macrophages, myofibroblasts, matrix, mechanics, and miscommunication [32], suggesting that interaction between the matrix and HSCs might play a crucial role in hepatic fibrogenesis. As a component of ECM, matricellular protein SPOCK1 acts as an oncogene in various cancers, including hepatocellular carcinoma, esophageal squamous cell carcinoma, colorectal cancer, gallbladder cancer, breast cancer, lung cancer, prostate cancer [12,13,14,15,16,17, 33], etc. The oncogenic role of SPOCK1 is primarily achieved by inducing proliferation, invasion, and inhibiting apoptosis. In this study, we demonstrated that SPOCK1 exerted a profound pro-fibrotic role in liver fibrosis.
Several cytokines are involved in pro-fibrogenic HSC responses, such as TGF-β1, PDGF, CTGF, and VEGF [5]. Our data demonstrate that TGF-β1 and PDGF-BB both upregulated SPOCK1 expression in HSCs, and this result is consistent with studies that SPOCK1 is a target gene of TGF-β1 [16, 17]. However, we found that PDGF-BB exerted a stronger effect on SPOCK1 expression in HSCs, which attracted our attention and impelled us to investigate the underlying mechanism; subsequently, we confirmed that PDGF-BB upregulated SPOCK1 level by activating the PI3K/Akt/FoxM1 signaling pathway. Interestingly, a previous study reported that transgenic expression of FoxM1b induces liver fibrosis in mice [34]. Our results are consistent with the reported role of FoxM1 in liver fibrosis. Several studies have shown that PDGF-BB promotes HSC activation, proliferation, and migration [6, 26, 27]. By gain- and loss-of-function assays, we found that SPOCK1 not only mediated the function of PDGF-BB on HSCs but also promoted pro-fibrogenic HSC responses on its own, suggesting a crucial role for SPOCK1 in liver fibrosis. Congruently, in vivo, when HSC-expressed SPOCK1 was selectively knockdown, the collagen deposition, markers of HSC activation, proliferation, and migration all decreased tremendously.
Next, we made further exploration to elucidate the underlying mechanism how SPOCK1 activated the intracellular signaling pathways. As secreted proteins, matricellular proteins exert their roles mainly by interacting with integrins and subsequently activating the downstream pathways [7]. Previous studies have shown that osteopontin binds to integrin αvβ3 and that periostin interacts with integrin αv on HSCs [28, 29]. By performing co-IP, double IF staining, we found that SPOCK1 interacted with integrin α5β1 on HSCs and activated the intracellular PI3K/Akt signaling pathway. As previously reported, integrin α5β1 is a fibronectin receptor and is positively associated with HSC activation [35]. Interestingly, several studies have demonstrated that SPARC binds to integrin α5 or integrin β1 on skeletal muscle progenitor cell, hepatocytes, murine lens epithelial cells, and adipose stromal cells [36,37,38,39], thus, the structural similarity between SPOCK1 and SPARC might account for these results.
According to previous studies, SPOCK1 regulates the PI3K/Akt, Wnt/β-catenin and mTOR-S6K signaling pathways, and SPOCK1 promotes epithelial–mesenchymal transition [13,14,15, 40, 41]. In this study, we primarily focused on the PI3K/Akt signaling pathway, as it is not only a vital pathway responsible for liver fibrosis but also a common downstream pathway of SPOCK1, PDGF-BB, and integrins. We confirmed that the PI3K/Akt signaling pathway is critical for both PDGF-BB-induced SPOCK1 expression and SPOCK1-induced HSC activation, proliferation, and migration. There might be a positive feedback loop between the PI3K/Akt signaling pathway and SPOCK1. However, whether SPOCK1 regulates other signaling pathways in HSCs may require further exploration.
In summary, we have established that SPOCK1, which is upregulated by PDGF-BB by activating the PI3K/Akt/FoxM1 signaling pathway, promotes pro-fibrogenic HSC responses, thus promoting liver fibrosis. Moreover, SPOCK1 exerts its role by activating the integrin α5β1/PI3K/Akt signaling pathway. HSC-specific SPOCK1 knockdown tremendously attenuates liver fibrosis, potentially by inhibiting HSC activation, proliferation, and migration (Fig. 6f). Furthermore, this study reports a new role of SPOCK1 in liver fibrosis, which may be a potential interventional target for liver fibrosis.
References
Bataller R, Brenner DA. Liver fibrosis. J Clin Investig. 2005;115:209–18.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71.
Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Investig. 2013;123:1887–901.
Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411.
Borkham-Kamphorst E, Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 2016;28:53–61.
Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14.
Wu T, Ouyang G. Matricellular proteins: multifaceted extracellular regulators in tumor dormancy. Protein Cell. 2014;5:249–52.
Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–8.
Edgell CJBM, Marr HS. Testican-1: a differentially expressed proteoglycan with protease inhibiting activities. Int Rev Cytol. 2004;236:101–22.
Bocock JP, Edgell CJ, Marr HS, Erickson AH. Human proteoglycan testican-1 inhibits the lysosomal cysteine protease cathepsin L. Eur J Biochem. 2003;270:4008–15.
Li Y, Chen L, Chan THM, Liu M, Kong KL, Qiu JL, et al. SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology. 2013;144:179–91.e4.
Song X, Han P, Liu J, Wang Y, Li D, He J, et al. Up-regulation of SPOCK1 induces epithelial-mesenchymal transition and promotes migration and invasion in esophageal squamous cell carcinoma. J Mol Histol. 2015;46:347–56.
Zhao P, Guan HT, Dai ZJ, Ma YG, Liu XX, Wang XJ. Knockdown of SPOCK1 inhibits the proliferation and invasion in colorectal cancer cells by suppressing the PI3K/Akt pathway. Oncol Res. 2016;24:437–45.
Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao Y, et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer. 2015;14:12.
Fan LC, Jeng YM, Lu YT, Lien HC. SPOCK1 is a novel transforming growth factor-beta-induced myoepithelial marker that enhances invasion and correlates with poor prognosis in breast cancer. PLoS One. 2016;11:e0162933.
Miao L, Wang Y, Xia H, Yao C, Cai H, Song Y. SPOCK1 is a novel transforming growth factor-beta target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2013;440:792–7.
Atorrasagasti C, Aquino JB, Hofman L, Alaniz L, Malvicini M, Garcia M, et al. SPARC downregulation attenuates the profibrogenic response of hepatic stellate cells induced by TGF-beta1 and PDGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G739–48.
Atorrasagasti C, Peixoto E, Aquino JB, Kippes N, Malvicini M, Alaniz L, et al. Lack of the matricellular protein SPARC (secreted protein, acidic and rich in cysteine) attenuates liver fibrogenesis in mice. PLoS One. 2013;8:e54962.
Koo JH, Lee HJ, Kim W, Kim SG. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2. Gastroenterology. 2016;150:181–93 e8.
Lin J, Chen A. Activation of peroxisome proliferator-activated receptor-gamma by curcumin blocks the signaling pathways for PDGF and EGF in hepatic stellate cells. Lab Investig. 2008;88:529–40.
Fan Y, Du Z, Steib CJ, Ding Q, Lu P, Tian D, et al. Effect of SEPT6 on the biological behavior of hepatic stellate cells and liver fibrosis in rats and its mechanism. Lab Investig. 2019;99:17–36.
Xia L, Huang W, Tian D, Zhu H, Zhang Y, Hu H, et al. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:600–12.
Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, et al. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149:1053–67 e14.
Woodhoo A, Iruarrizaga-Lejarreta M, Beraza N, Garcia-Rodriguez JL, Embade N, Fernandez-Ramos D, et al. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology. 2012;56:1870–82.
Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–73.
Shah R, Reyes-Gordillo K, Rojkind M. Thymosin beta4 inhibits PDGF-BB induced activation, proliferation, and migration of human hepatic stellate cells via its actin-binding domain. Expert Opin Biol Ther. 2018;18:177–84.
Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology. 2012;55:594–608.
Sugiyama A, Kanno K, Nishimichi N, Ohta S, Ono J, Conway SJ, et al. Periostin promotes hepatic fibrosis in mice by modulating hepatic stellate cell activation via alphav integrin interaction. J Gastroenterol. 2016;51:1161–74.
Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68-69:435–51.
Schnittert J, Bansal R, Storm G, Prakash J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev. 2018;129:37–53.
Pakshir P, Hinz B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68-69:81–93.
Chien MH, Lin YW, Wen YC, Yang YC, Hsiao M, Chang JL, et al. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J Exp Clin Cancer Res. 2019;38:246.
Park HJ, Gusarova G, Wang Z, Carr JR, Li J, Kim KH, et al. Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol Med. 2011;3:21–34.
Milliano MT, Luxon BA. Initial signaling of the fibronectin receptor (α5β1 integrin) in hepatic stellate cells is independent of tyrosine phosphorylation. J Hepatol. 2003;39:32–7.
Nakamura K, Yamanouchi K, Nishihara M. Secreted protein acidic and rich in cysteine internalization and its age-related alterations in skeletal muscle progenitor cells. Aging Cell. 2014;13:175–84.
Aseer KR, Silvester AJ, Kumar A, Choi MS, Yun JW. SPARC paucity alleviates superoxide-mediated oxidative stress, apoptosis, and autophagy in diabetogenic hepatocytes. Free Radic Biol Med. 2017;108:874–95.
Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–37.
Nie J, Chang B, Traktuev DO, Sun J, March K, Chan L, et al. IFATS collection: combinatorial peptides identify alpha5beta1 integrin as a receptor for the matricellular protein SPARC on adipose stromal cells. Stem Cells. 2008;26:2735–45.
Wang T, Liu X, Tian Q, Liang T, Chang P. Reduced SPOCK1 expression inhibits non-small cell lung cancer cell proliferation and migration through Wnt/beta-catenin signaling. Eur Rev Med Pharmacol Sci. 2018;22:637–44.
Wang Y, Wang W, Qiu E. SPOCK1 promotes the growth of osteosarcoma cells through mTOR-S6K signaling pathway. Biomed Pharmacother. 2017;95:564–70.
Acknowledgements
This research was funded by grants from the National Key Research and Development Program of China 2018YFC1312103 (LX), National Natural Science Foundation of China No. 81972237 (LX), No. 81772623 (LX), No. 81772610 (DT), and No. 81974071 (DT).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Du, Z., Lin, Z., Wang, Z. et al. SPOCK1 overexpression induced by platelet-derived growth factor-BB promotes hepatic stellate cell activation and liver fibrosis through the integrin α5β1/PI3K/Akt signaling pathway. Lab Invest 100, 1042–1056 (2020). https://doi.org/10.1038/s41374-020-0425-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-0425-4
This article is cited by
-
N-Acetyl-L-cysteine facilitates tendon repair and promotes the tenogenic differentiation of tendon stem/progenitor cells by enhancing the integrin α5/β1/PI3K/AKT signaling
BMC Molecular and Cell Biology (2023)
-
Engineered fibrotic liver-targeted truncated transforming growth factor β receptor type II variant for superior anti-liver fibrosis therapy
Archives of Pharmacal Research (2023)
-
High-dimensional hepatopath data analysis by machine learning for predicting HBV-related fibrosis
Scientific Reports (2021)