Abstract
Allergic asthma is one of the most common immune-mediated disorders affecting the lungs. It is characterized clinically by airway hyperresponsiveness, eosinophilia, enhanced IL-4 and IL-13, peribronchial inflammation with mononuclear cell infiltration, and goblet cell hyperplasia associated with increased mucus production. However, chronic asthma with repeated exposures to inhaled allergens can result in subepithelial pulmonary fibrosis. The transient receptor potential cation channel subfamily V member 4 (TRPV4) protein can promote the generation of myofibroblasts and pulmonary fibrosis. Here, we investigated the possibility that TPRV4 facilitates the development of allergic asthma and subsequent pulmonary fibrosis in the lung. To test this, wild-type (WT) and TPRV4 gene knockout (KO) mice were repeatedly sensitized with chicken ovalbumin (OVA) and repeatedly subjected to aerosol challenge with 1% OVA. We found that there were no significant differences in the development of allergic asthma between the WT and TPRV4 KO mice. Both groups of mice exhibited similar levels of airway hyperresponsiveness, IL-13, IL-5, OVA-specific IgE, eosinophilia, mucus-secreting goblet cell hyperplasia, and deposition of collagen fiber, which is a hallmark of the pulmonary fibrosis. Thus, these data suggest that TPRV4 protein is dispensable in the initiation and development of airway asthma and subsequent fibrosis.
Similar content being viewed by others
Introduction
Allergic asthma is one of the most common noncommunicable diseases of the pulmonary system, affecting an estimated 334 million people of all age groups worldwide [1]. Allergic asthma is characterized by difficulty in breathing, shortness of breath, chest pain, and wheezing. Bacterial, viral, and fungal respiratory infections predispose the respiratory system to allergic inflammation, and exacerbate the symptoms of allergic asthma [2,3,4,5,6,7,8,9,10,11]. Allergic asthma is characterized as glucocorticoid-sensitive mild to moderate asthma or as glucocorticoid-insensitive, difficult to treat, severe asthma. In mild to moderate asthma, airway inflammation is chronic, characterized by an abnormal shift toward a T helper 2 (Th2) -mediated immune response. Th2 driven allergic inflammation is characterized by elevated expression of interleukin-4 (IL-4), IL-13, and IL-5, along with airway eosinophilia, augmented allergen-specific IgE, airway hyperresponsiveness, goblet cell hyperplasia, and peribronchial inflammation with infiltration of mononuclear cells [2,3,4,5,6,7,8,9,10,11]. Severe asthma is characterized by type 2-mediated eosinophilic inflammation or by mixed inflammation with neutrophilic and eosinophilic infiltration, both of which are corticosteroid insensitive and difficult to treat and control with conventional methods [8].
The transient receptor potential vanilloid 4 (TRPV4) is a calcium permeant, nonselective cation channel protein involved in osmotic, thermal, and mechanosensitivity [12,13,14,15,16,17,18,19,20]. TRPV4 proteins are activated by a variety of physical and chemical stimuli and is expressed in a wide range of cell types, including neurons, keratinocytes, endothelial cells, and epithelial cells, and in specific tissues including heart, lung, kidney, cartilage, and bone [12,13,14,15,16,17,18,19,20]. TRPV4 proteins are expressed in human airway smooth muscle cells, where they function as an osmolarity sensor for calcium influx [20]. TRPV4 was predicted to have a functional role in the pathophysiology of bronchial hyperresponsiveness in asthma [21]. Recently, the TRPV4 was found to be expressed in lung fibroblasts contributing to the development of pulmonary fibrosis [12]. Moreover, TRPV4 knockout (KO) mice were found to be protected from experimentally induced pulmonary fibrosis [12]. However, the functional role of TRPV4 gene in the development and pathogenesis of allergic asthma remains elusive. Here, we found that TRPV4 KO and wild-type (WT) mice that were sensitized and then challenged with the allergen chicken ovalbumin (OVA) showed no significant differences in various parameters of asthma including IL-4, IL-5, IL-13, OVA-specific IgE, airway hyperresponsiveness, eosinophilia, airway inflammation, goblet cell hyperplasia, or peribronchial collagen deposition. These findings indicate that TRPV4 is dispensable for the initiation and development of allergic asthma.
Materials and methods
Antibodies, mice, and reagents
Chicken egg ovalbumin (OVA, premium quality Grade V) was obtained from Sigma-Aldrich (St. Louis, MO). A TRPV4 KO mouse line on a C57BL/6 background was generated by Dr. Makoto Suzuki (Jichi Medical University, Tochigi, Japan) as described previously [22]. Inbred C57BL/6 mice (six–eight-week old) were obtained from Charles River (Wilmington, MA). Both male and female WT and TRPV4 KO mice were used in the experiments. The Institutional Animal Care and Use Committee (IACUC) of the University of Maryland reviewed and approved all animal research pertaining to this project, and all the animals were housed in HEPA filtered animal cages and provided ad libitum access to food and water.
APC-Cy7 rat anti-mouse CD45, APC-Cy7 rat IgG2b, FITC hamster anti-mouse CD11c, FITC Ar hamster IgG1, PE rat anti-mouse siglec-F, PE-rat IgG2a, APC-Cy7 rat anti-mouse Ly6G and Ly6C (Gr1), and APC-Cy7 rat IgG2b were purchased from BD Pharmingen (San Diego, CA); APC anti-mouse/human CD11b, APC rat IgG2b, and PE rat anti-mouse F4/80 were purchased from Biolegend (San Diego, CA).
Isolation of lung mononuclear cells
Lung mononuclear cells (MNCs) were isolated following protocols as described previously [23,24,25]. Briefly, after sacrificing the mice with an excessive injectable anesthetic Avertin, the lungs were collected aseptically and minced into small pieces and digested with a mixture of enzymes containing dispase (2.4 units/ml in DMEM), pronase (1.5 mg/ml in DMEM), and DNase I (0.5 mg/ml) for 30 min at 37 °C.
Enzyme-linked immunosorbent assay (ELISA)
OVA-specific IgE concentrations in serum and BAL were determined by ELISA as previously described [23,24,25]. The ELISA plate was coated with ovalbumin (2 μg/ml) overnight at 4 °C. The ELISA plate was then washed three times with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBST) and blocked with 200 μl of blocking solution (10% fetal bovine serum (FBS) in PBS) for 1 h at room temperature. The ELISA plates were again washed three times with PBST, and then incubated with 100 μl of OVA-specific IgE standards or with diluted serum samples for 2 h at room temperature. ELISA plates were washed five times with PBST, and then further incubated for 1 h at room temperature with 100 μl of biotinylated anti-mouse IgE (1:250, BD) and HRP-conjugated Streptavidin (1:250, BD). Finally, the ELISA plates were washed seven times, developed with ABTS, and absorbance was measured on a Victor III microplate reader (PerkinElmer). Concentrations of mouse IL-4 (BD, San Diego, CA), mouse IL-5 (BD, San Diego, CA), and mouse IL-13 (eBioscience, San Diego, CA) in serum and BAL were measured by ELISA following the manufacturer’s instructions.
Flow cytometry
Eosinophils and neutrophils present in the BAL and lung mononuclear cells were analyzed by the flow cytometry. Isolated cells were first incubated with Fcγ receptor block solution containing anti-CD16/32 Fc block (BD, San Diego, CA). Pooled cells from 4 to 5 mice were washed three times with FACS buffer (2% FBS in PBS), and analyzed for surface expression of CD11b, CD45, CD11c, Siglec-F, F4/80, and Gr1. The cells were incubated with the antibodies APC-Cy7 rat anti-mouse CD45, FITC hamster anti-mouse CD11c, PE rat anti-mouse Siglec-F, APC anti-mouse/human CD11b, PE rat anti-mouse F4/80, APC-Cy7 rat anti-mouse Ly6G, and Ly6C (Gr1) for 30 min at 4 °C. Control cells were similarly incubated with the corresponding isotype-matched control antibodies. The cells were then washed three times with FACS buffer, resuspended, and analyzed using on a FACSAria II (BD, San Diego, CA). Data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Ovalbumin sensitization and challenge
Ovalbumin sensitization and challenge were performed following a sensitization protocol with chicken OVA and alum as described previously [23, 26,27,28,29,30]. C57BL/6 wild-type and TRPV4 KO mice were sensitized with an allergen by intraperitoneal injection of 100 μl PBS containing 100 μg OVA and 4 mg aluminum hydroxide (Imject Alum Adjuvant, Thermo Fisher Scientific, Waltham, MA) on day zero. On day 7 and day 14, the mice were again sensitized with the same dose of OVA without adjuvant. Mice were challenged with 1% nebulized OVA on consecutive days from day 21 to 26 for 30 min. On day 27, airway hyperresponsiveness was measured, and the mice were euthanized on day 28 with excessive injectable anesthetic Avertin. Control WT and TRPV4 KO mice were left untreated and sacrificed on the day of the experiment. Lung tissue samples were collected for histopathology analysis and for the isolation of lung mononuclear cells. Serum and BAL fluids were collected. The cells were separated from the BAL fluid. The lung mononuclear and BAL cells were used for flow cytometry and cytospin analysis. Differential cell count analysis was performed by staining with Hema-3 stain (Thermo Fisher Scientific, Waltham, MA).
Measurement of AHR
Airway hyperresponsiveness (AHR) was measured 24 h after the last aerosol challenge in conscious, unrestrained mice using whole-body plethysmography (EMKA Technologies, Paris, France) following procedures as described previously [25, 31]. Baseline AHR values were taken first, and response to OVA allergen was then measured following exposure to OVA via nebulized PBS solution. Mice were subsequently exposed to increasing concentration of β-methacholine (3–100 mg/ml) (MilliporeSigma), and AHR was measured. Nebulization with various reagents was carried out for 2 min, an AHR reading was recorded for 2 min after each nebulization. Average values for three measurements are presented as absolute Penh values.
Histopathology
Forty-eight hours after the last aerosol OVA challenge, the mice were euthanized. For control mice, naive WT and TRPV4 KO mice were also euthanized. Lung tissues were collected and fixed in 10% buffered formalin. Thin sections of paraffin-embedded, formalin-fixed tissue were stained with hematoxylin and eosin (H&E), periodic acid-schiff (PAS), and Masson-trichrome stains. Stained sections were examined by light microscopy (Carl Zeiss, NY). Images were captured using a Zeiss Axiocam 105. Average inflammation scores determined from H&E stained sections were recorded as described previously [25, 31]. Mucus-secreting goblet cells were analyzed on PAS stained sections as described previously [25, 31]. Collagen deposition/fibrosis in the lung were determined from Masson-trichrome staining.
Statistics
Two-way ANOVA was used to evaluate statistical differences between different treatment groups for airway hyperresponsiveness measurement in Fig. 1 and Student’s t test was used for all other experimental analysis. A p-value < 0.05 was considered significant. GraphPad Prism 5 software was used for the statistical analysis.
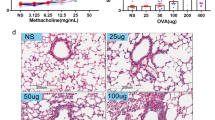
TRPV4 gene is not involved in airway hyperresponsiveness. a Graphical representation of chicken ovalbumin-based airway allergy model. C57BL/6 WT and TRPV4 KO mice (6–8-week old) were sensitized and challenged with OVA allergen. On day zero, mice were sensitized with 100 μg OVA combined with 4 mg of Alum via intraperitoneal injection. On day 7 and 14, 100 μg of OVA in PBS was given intraperitoneally. From day 21 to 26, the mice were challenged with nebulized 1% OVA in PBS. AHR was measured 24 h (day 27) after the last OVA challenge, and on day 28, the mice were euthanized and the lung, BAL, and blood were collected for further analysis. b TRPV4 KO and wild-type mice were sensitized and challenged with OVA. Baseline AHR was measured 24 h after the last OVA challenge and following 2 min exposure to PBS (vehicle control) or incremental doses of β-methacholine in PBS (3–100 mg/ml). Airway resistance is expressed as PenH. Data are mean ± SEM of four individual mice
Results
TRPV4 is not involved in the development of airway hyperresponsiveness
Mouse models of asthmatic inflammation using chicken OVA as an allergen have been well characterized [23, 26,27,28,29,30]. Following intraperitoneal injection of OVA mixed with alum and subsequent challenge with OVA, allergic inflammation develops, which is characterized by BAL and lung tissue eosinophilia, airway remodeling, induction of Th2 cytokines, and AHR. As detailed in “Methods” section, we have generated a mouse model of developing airway allergic inflammation in WT and TRPV4 KO mice (Fig. 1a). In this model, we found that even though OVA-sensitized and challenged TRPV4 KO mice had slightly higher AHR in response to increasing doses of β-methacholine compared with WT mice, there were no significant differences in airway resistance between these groups of mice (Fig. 1b). These results indicate that the expression of TRPV4 is not required for initiation or development of AHR during the asthmatic episode.
TRPV4 KO and WT mice express similar levels of allergen specific IgE and Th2 cytokines
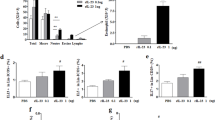
TRPV4 KO and WT mice sensitized and challenged with OVA developed similar levels of OVA allergen specific IgE in both serum and BAL (Fig. 2a). IL-5 is responsible for the development of allergic eosinophilia and IL-13 is involved in the development of airway hyperresponsiveness and mucus hypersecretion. Likewise, the induction of Th2 cytokines including IL-5 and IL-13 were not significantly altered between WT and TRPV4 KO mice (Fig. 2b, c), although the TRPV4 KO mice had slightly lesser IL-5 (Fig. 2b). Moreover, IL-4 in both serum and BAL of WT and TRPV4 KO mice were not detectable by ELISA. As expected, both untreated WT and TRPV4 KO control mice had no detectable OVA-specific IgE, IL-4, and IL-5 in the BAL and serum; IL-13 was not detected in BAL and significantly reduced in serum (Fig. 2c) when compared with OVA-sensitized and challenged mice. These results suggest that loss of TRPV4 expression has no effect on the levels of Th2 cytokine or OVA-specific IgE responses.
Analysis of Th2 cytokines and OVA-specific IgE in BAL and serum. a–c WT and TRPV4 KO mice were sensitized and challenged with OVA. The mice were euthanized, and BAL and serum were collected 48 h after last aerosol challenge and analyzed by ELISA for Th2 cytokines IL-5, IL-13, and OVA-specific IgE. Data are mean ± SEM of four to six mice and are representative of three independent experiments. The untreated WT and TRPV4KO control mice were sacrificed, and the BAL and serum were collected and analyzed by ELISA for the presence of OVA-specific IgE, IL-4, IL-5, and IL-13. Data are mean ± SEM of five WT and TRPV4 KO control mice
TRPV4 KO and WT mice develop similar levels of eosinophilia in the lung
Flow cytometric analysis of BAL cells and lung MNC showed that OVA-sensitized and challenged TRPV4 KO and WT mice developed similar levels of eosinophilia. In BAL from WT mice, 88% of MNCs were eosinophils compared with 81% of MNCs in BAL from TRPV4 KO mice. Similarly, 55% of MNCs from the lung of WT mice were eosinophils compared with 57% of MNCs from the lung of TRPV4 KO mice (Fig. 3a, b). Similarly, there were no significant differences in the levels of neutrophils between the TRPV4 KO and WT mice. Both groups of mice exhibited comparable levels of neutrophils in BAL and lung MNCs (Fig. 3c, d). These results were confirmed by differential cell count analysis of Hema-3 stained BAL and lung mononuclear cells, which showed that eosinophils were the major population of MNCs in both WT and TRPV4 KO mice, and that there were no significant differences in eosinophil counts between TRPV4 KO and WT mice (Fig. 4a, b). As expected, by using flow cytometric analysis, both untreated WT and TRPV4 KO control mice had a significantly reduced amount of eosinophils and neutrophils in both BAL and Lung MNC when compared with OVA-sensitized and challenged mice (Fig. 3e–h). These results were further confirmed by differential cell count analysis by Hema-3 staining of BAL and lung mononuclear cells, where eosinophils were not detected in the BAL and lung MNC, while neutrophils were not detected in the BAL (Fig. 4c, d) in these control mice. These results suggest that kinetics of the inflammatory process of allergic asthma is not substantially impacted by TRPV4 expression.
Flow cytometric analysis of eosinophils and neutrophils. a–d Top panel. WT and TRPV4 KO mice were sensitized and challenged with OVA. The mice were euthanized 48 h after the last OVA challenge, and the BAL and lung mononuclear cells were collected. The cells were surface stained for various markers with specific and isotype control antibodies: CD11c, CD11b, F4/80, Gr1, Siglec F, and CD45. Total MNCs were gated either on CD45+CD11b+ and CD11c−SiglecF+ to identify eosinophils or were gated on CD11c−CD11b+ and F4/80−Gr1+ to identify neutrophils. The BAL and lung MNCs were separately pooled from three individual mice. Data shown are representative of three independent experiments. Bottom Panel. Quantification of eosinophils and neutrophils in the BAL and lung mononuclear cells. Separate pools of BAL and lung MNCs were prepared from three individual mice. Data shown are representative of three independent experiments. e–h Top panel. Eosinophils and neutrophils were detected by flow cytometry analysis from BAL and lung MNCs of WT and TRPV4 control mice using a similar gating strategy as described above. Data are mean ± SEM of five WT and TRPV4 KO control mice. Bottom panel. Quantification of eosinophils and neutrophils in BAL and lung mononuclear cells. Data are mean ± SEM of five WT and TRPV4KO control mice
Differential cells count analysis of BAL and lung mononuclear cells. a, b WT and TRPV4 KO mice were sensitized with OVA and challenged with nebulized OVA. The mice were euthanized 48 h after the last OVA challenge and the BAL and lung MNCs were isolated. The cells were centrifuged onto glass slides by cytospin, air-dried, and stained with Hema-3 stain. a Representative images for Hema-3 stains from each group are shown. b Differential cell count analysis of leukocytes present in the BAL and lung MNCs were determined. For a, b the BAL and lung MNCs from three individual mice were separately pooled. Data shown are representative of three independent experiments. c, d The untreated WT and TRPV4KO control mice were sacrificed and the BAL and lung MNCs were collected, stained, and analyzed as described above. c Representative images for Hema-3 stains from each control group are shown. d Differential cell count analysis of leukocytes present in BAL and lung MNCs were determined. Data are mean ± SEM of five WT and TRPV4 KO control mice
TRPV4 KO and WT mice exhibit similar levels of allergic inflammation in the lung
Lung tissue sections obtained from WT controls and TRPV4 KO mice showed normal lung architecture (Fig. 5a, d, g). The untreated WT and TRPV4 KO control mice showed barely detectable inflammation by H&E staining (Fig. 5c), less number of goblet cells by PAS staining (Fig. 5f) and a much lesser amount of collagen deposition (indicated by blue color) by Masson-trichrome staining (Fig. 5i) when compared with OVA-sensitized and challenged mice. Histopathological analysis of H&E stained sections of lung tissue from OVA-sensitized and challenged TRPV4 KO and WT mice showed comparable inflammatory changes with eosinophilic and neutrophilic infiltrations, bronchiolar epithelial hyperplasia, and clearly visualized collagen deposition (Fig. 5b). Pathological scores for allergic inflammatory changes were similar between these two groups (Fig. 5c). PAS staining analysis of lung tissue showed that the level of mucus-secreting goblet cell hyperplasia was comparable between WT and TRPV4 KO mice (Fig. 5e). Percentages of mucus-secreting goblet cells were also similar between WT and TRPV4 KO mice (Fig. 5f). Likewise, Masson-trichrome staining of lung tissue sections showed that fibrosis of the lung around the bronchioles, indicated by deposition of collagen, was similar between the WT and TRPV4 KO mice (Fig. 5h). Quantification of collagen deposition indicated by the area of blue staining in Fig. 5h was not significantly different between WT and TRPV4 KO mice that were sensitized and challenged by OVA (Fig. 5i). These results indicate that TRPV4 expression is not involved in the development of allergic inflammation in the lung.
Histopathological analysis of airway inflammation. a–i WT and TRPV4 KO mice were OVA-sensitized and challenged with OVA. Experimental mice and untreated control WT and TRPV4 KO mice were euthanized 48 h after last OVA challenge and lung tissues were collected, fixed in buffered formalin, paraffin-embedded, and thin sections were made. Sections were stained with H&E (a, b), periodic acid schiff (d, e), or Masson trichrome (g, h). c, f, i Quantified data (mean ± SEM) from analysis of thin sections prepared from three individual OVA-sensitized and challenged mice stained with H&E (c), periodic acid schiff (f), or Masson trichrome (i). Images are representative of three independent experiments (b, e and h). Similarly, lung sections from untreated WT and TRPV4 KO control mice were also prepared. Quantified data (mean ± SEM) from analysis of thin sections prepared from five individual WT and TRPV4 KO control mice stained with H&E (c), periodic acid schiff (f), or Masson trichrome (i). Data are mean ± SEM of five WT and TRPV4 KO control mice (c, f, i). Image is representative of five individual mice. a, d, g The percentage area of blue color in Masson-trichrome stain for deposition of collagen/lung fibrosis was estimated using ImageJ software
Discussion
Allergic asthma is an immune-mediated disorder affecting the respiratory tract, which is characterized by airway remodeling, excessive mucus secretion occluding the airways, increased airway resistance and inflammation, and infiltration of immune cells in the airways [2,3,4,5,6,7,8,9,10,11]. The pathological events and the underlying causes leading to the development of allergic asthma are multifactorial in nature. Environmental factors such as repeated exposure to a variety of allergens and host factors including the presence of susceptibility genes predispose the individual to the development of allergic asthma [2,3,4,5,6,7,8,9,10,11]. Th2 mediated allergic inflammation, which includes eosinophilia, goblet cell hyperplasia, increased AHR, induction of Th2 cytokines IL-4, IL-5, and IL-13, is a common feature of moderate asthma. Severe asthma is characterized by both Th2-mediated eosinophilic allergic asthma and Th1- or Th17-mediated neutrophilic allergic inflammation of the lung [2,3,4,5,6,7,8,9,10,11]. Mouse models of the development of allergic asthma using OVA allergen are well characterized and can mimic the symptoms and changes associated with human Th2-based airway allergy.
TRPV4 is known to be expressed in human and mouse lung fibroblasts, and TRPV4 KO mice are protected from the development of lung fibrosis and deposition of the collagen fiber in the inflamed lung of the bleomycin-treated mice [12]. TRPV4 is expressed in the human airway smooth muscle cells, and is predicted to have a functional role in the development of asthma [20, 21]. Allergic asthma also shares a common feature of the development of lung fibrosis and deposition of collagen in the lung, and thus we rationalized that mice lacking TRPV4 would also be protected from airway allergic asthma. However, when we tested this hypothesis in the OVA-based allergic asthma model, we found no significant differences compared with WT mice. In TRPV4 KO mice, enhancement of AHR, induction of Th2 cytokines, OVA allergen-specific IgE, eosinophilia of the lung and BAL, the development of airway allergic inflammation, increased mucus production, goblet cell hyperplasia, and deposition of collagen in lung tissue were unaffected, indicating that the TRPV4 gene is dispensable for the development of OVA-induced allergic inflammation. Furthermore, results from our untreated control mice provided strong evidence that our OVA-induced sensitization succeeded, although there was no difference in various parameters of asthma between WT and TRPV4 KO group. We found that the untreated WT and TRPV4 KO control mice had the normal features of lung architecture, with no detectable OVA-specific IgE and IL-5 and barely visible eosinophils and neutrophils in BAL and serum.
Several questions may still exist. First, Cantero-Recasens et al. showed there is no significant association between known TRPV4 single nucleotide polymorphisms (SNPs) (TRPV4-P19S) and asthma symptoms and risks like cough/wheeze [32]. However, we are unable to rule out the possibility that there are other asthma symptom risks (e.g. for exacerbation) linked to TRPV4 SNPs which have not been explored. Second, it is interesting to know whether the TRPV4 is also unessential for the development of asthmatic inflammation in house dust mite-based mouse asthma model. Recently, it has been reported that TRPV4 KO mice are protected from dust mite-induced airway remodeling; suggesting TRVP4 contributes to pathologic airway remodeling in asthma [33]. However, in our experimental analysis of OVA-based model, we found that TRPV4 KO mice were not protected from allergic inflammation, this discrepancy might be due to the different types of allergen used in the experiments. More experiments should be performed to verify this difference. Third, allergic asthma can be divided into several stages from moderate to severe persistent asthma. Our OVA-based asthma mouse model in this study reflects a moderate asthmatic condition. It is also noteworthy to know whether TRPV4 plays a role in the course of severe persistent asthma. Fourth, although there are important gaps in understanding lung fibrosis in asthma, it is generally believed that lung fibrogenesis involves a dynamic interplay in multiple signaling pathways. The loss of TRPV4 function may not completely abolish other pathways that promote or induce the biosynthesis and deposition of extracellular matrix in asthma. Thus, we conclude that the TRPV4 gene plays a minimal or no role in the initiation and development of allergic inflammation in OVA-based mouse asthma model.
References
Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–78.
Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8.
Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–92.
Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17.
Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–30.
Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–98.
Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Crit Care. 2002;6:30–44.
Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–76.
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56.
Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–4.
Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–21.
Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest. 2014;124:5225–38.
Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101:396–401.
Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417.
Guler AD, Lee H, Lida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–14.
Gunthorpe MJ, Benham CD, Randall AD, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–91.
Liedtke W, Choe Y, Marti-Renom MA, Bell MA, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35.
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. TRPV4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2002;2:695–702.
Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103:2–17.
Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, et al. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–L278.
Liedtke W, Simon SA. A possible role for TRPV4 receptors in asthma. Am J Physiol Lung Cell Mol Physiol. 2004;287:L269–L271.
Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–8.
Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–20.
Jones T,G, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL9 and CD1d-restricted NKT cells. J Immunol. 2009;183:5251–60.
Palaniyandi S, Liu X, Periasamy S, Ma A, Tang J, Jenkins M, et al. Inhibition of CD23-mediated IgE transcytosis suppresses the initiation and development of allergic airway inflammation. Mucosal Immunol. 2015;8:1262–74.
Reddy AT, Lakshmi SP, Reddy RC. Murine model of allergen induced asthma. J Vis Exp. 2012;14:e3771.
Walsh ER, Thakar J, Stokes K, Huang F, Albert R, August A. Computational and experimental analysis reveals a requirement for eosinophil-derived IL-13 for the development of allergic airway responses in C57BL/6 mice. J Immunol. 2011;186:2936–49.
Li K, Zhang Y, Liang KY, Xu S, Zhou XJ, Tan K, et al. Rheb1 deletion in myeloid cells aggravates OVA-induced allergic inflammation in mice. Sci Rep. 2017;7:42655.
Warren KJ, Dickinson JD, Nelson AJ, Wyatt TA, Romberger DJ, Poole JA. Ovalbumin-sensitized mice have altered airway inflammation to agriculture organic dust. Respir Res. 2019;20:51.
Reddy AT, Lakshmi SP, Dornadula S, Pinni S, Rampa DR, Reddy RC. The nitrated fatty acid 10-nitro-oleate attenuates allergic airway disease. J Immunol. 2013;191:2053–63.
Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75.
Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem. 2010;285:27532–5.
Gombedza F, Kondeti V, Al-Azzam N, Koppes S, Duah E, Patil P, et al. Mechanosensitive transient receptor potential vanilloid 4 regulates Dermatophagoides farinae-induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB J. 2017;31:1556–70.
Acknowledgements
We are grateful for receiving the technical advice from Dr Yunsheng Wang, Dr Douglas A. Powell, Dr Weizhong Li, Dr Susan Park-Ochsner, Dr Xiaoyang Liu, Dr Gefei Wang, and Dr Dhurba P. Dhungana.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palaniyandi, S., Rajendrakumar, A.M., Periasamy, S. et al. TRPV4 is dispensable for the development of airway allergic asthma. Lab Invest 100, 265–273 (2020). https://doi.org/10.1038/s41374-019-0305-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-019-0305-y
This article is cited by
-
TRP channels associated with macrophages as targets for the treatment of obese asthma
Lipids in Health and Disease (2024)
-
Blocking group 2 innate lymphoid cell activation and macrophage M2 polarization: potential therapeutic mechanisms in ovalbumin-induced allergic asthma by calycosin
BMC Pharmacology and Toxicology (2024)