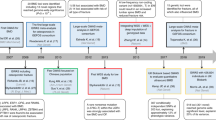
Key Points
-
The genetics of osteoporosis is a rapidly evolving field, which has been greatly expanded by increased understanding of the human genome
-
The list of identified loci associated with osteoporosis phenotypes is growing rapidly, and still incomplete
-
Studies of monogenic bone diseases and genome-wide association studies of complex traits have identified dozens of genes and loci that influence skeletal traits
-
Further knowledge of the genetics of osteoporosis will emerge from mining large databases containing a wealth of genomic information
-
Many candidate osteoporosis-related loci require detailed functional follow-up to understand the underlying biological mechanisms determining trait variation
-
Despite this wealth of new genetic information, its application to the clinical management of osteoporosis remains in its infancy
Abstract
Osteoporosis is characterized by low bone mass and an increased risk of fracture. Genetic factors, environmental factors and gene–environment interactions all contribute to a person's lifetime risk of developing an osteoporotic fracture. This Review summarizes key advances in understanding of the genetics of bone traits and their role in osteoporosis. Candidate-gene approaches dominated this field 20 years ago, but clinical and preclinical genetic studies published in the past 5 years generally utilize more-sophisticated and better-powered genome-wide association studies (GWAS). High-throughput DNA sequencing, large genomic databases and improved methods of data analysis have greatly accelerated the gene-discovery process. Linkage analyses of single-gene traits that segregate in families with extreme phenotypes have led to the elucidation of critical pathways controlling bone mass. For example, components of the Wnt–β-catenin signalling pathway have been validated (in both GWAS and functional studies) as contributing to various bone phenotypes. These notable advances in gene discovery suggest that the next decade will witness cataloguing of the hundreds of genes that influence bone mass and osteoporosis, which in turn will provide a roadmap for the development of new drugs that target diseases of low bone mass, including osteoporosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
22 July 2016
In Table 1 of this Review, the protein names and protein functions for TNFSF11 and TNFRSF11A were incorrectly associated. The entries have been corrected and the protein function for TNFRSF11A has been clarified in the online version of the article. The authors apologize for this error.
References
Smith, D. M. et al. Genetic factors in determining bone mass. J. Clin. Invest. 52, 2800–2808 (1973).
Pocock, N. A. et al. Genetic determinants of bone mass in adults. A twin study. J. Clin. Invest. 80, 706–710 (1987).
Hernandez-de Sosa, N. et al. Heritability of bone mineral density in a multivariate family-based study. Calcif. Tissue Int. 94, 590–596 (2014).
Morrison, N. A., Yeoman, R., Kelly, P. J. & Eisman, J. A. Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor gene polymorphism and circulating osteocalcin. Proc. Natl Acad. Sci. USA 89, 6665–6669 (1992).
Morrison, N. A. et al. Prediction of bone density from vitamin D receptor alleles. Nature 367, 284–287 (1994).
Riggs, L. B. et al. The contribution of vitamin D receptor gene alleles to the determination of bone mineral density in normal and osteoporotic women. J. Bone Miner. Res. 10, 991–996 (1995).
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–795 (2001).
Ioannidis, J. P. et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA 292, 2105–2114 (2004).
Uitterlinden, A. G. et al. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann. Intern. Med. 145, 255–264 (2006).
Ralston, S. H. et al. Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med. 3, e90 (2006).
Langdahl, B. L. et al. Large-scale analysis of association between polymorphisms in the transforming growth factor beta 1 gene (TGFB1) and osteoporosis: the GENOMOS study. Bone 42, 969–981 (2008).
van Meurs, J. B. et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299, 1277–1290 (2008).
Xie, W., Ji, L., Zhao, T. & Gao, P. Identification of transcriptional factors and key genes in primary osteoporosis by DNA microarray. Med. Sci. Monit. 21, 1333–1344 (2015).
Gusev, A. et al. Partitioning heritability of regulatory and cell-type-specific variants across 11 common diseases. Am. J. Hum. Genet. 95, 535–552 (2014).
Adams, D. J. & Ackert-Bicknell, C. L. Genetic regulation of bone strength: a review of animal model studies. BoneKEy Rep. 4, 714 (2015).
Wu, S. et al. Genome-wide approaches for identifying genetic risk factors for osteoporosis. Genome Med. 5, 44 (2013).
Burgers, T. A. & Williams, B. O. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone 54, 244–249 (2013).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013).
Gong, Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001).
Little, R. D. et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 70, 11–19 (2002).
Boyden, L. M. et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346, 1513–1521 (2002).
Brunkow, M. E. et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589 (2001).
Balemans, W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 (2001).
Balemans, W. et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 39, 91–97 (2002).
Staehling-Hampton, K. et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am. J. Med. Genet. 110, 144–152 (2002).
Richards, J. B. et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371, 1505–1512 (2008).
van Meurs, J. J. et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299, 1277–1290 (2008).
Styrkarsdottir, U. et al. New sequence variants associated with bone mineral density. Nat. Genet. 41, 15–17 (2009).
Keupp, K. et al. Mutations in WNT1 cause different forms of bone fragility. Am. J. Hum. Genet. 92, 565–574 (2013).
Laine, C. M. et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 368, 1809–1816 (2013).
Pyott Shawna, M. et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am. J. Hum. Genet. 92, 590–597 (2013).
Fahiminiya, S. et al. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 50, 345–348 (2013).
Palomo, T. et al. Skeletal characteristics associated with homozygous and heterozygous WNT1 mutations. Bone 67, 63–70 (2014).
Joeng, K. S. et al. The swaying mouse as a model of osteogenesis imperfecta caused by WNT1 mutations. Hum. Mol. Genet. 23, 4035–4042 (2014).
Estrada, K. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 44, 491–501 (2012).
Zheng, H.-F. et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 8, e1002745 (2012).
Moayyeri, A. et al. Genetic determinants of heel bone properties: genome-wide association meta-analysis and replication in the GEFOS/GENOMOS consortium. Hum. Mol. Genet. 23, 3054–3068 (2014).
Medina-Gomez, C. et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 8, e1002718 (2012).
Kemp, J. P. et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS Genet. 10, e1004423 (2014).
Koller, D. L. et al. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J. Bone Miner. Res. 28, 547–558 (2013).
García-Ibarbia, C. et al. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos. Int. 24, 2449–2454 (2013).
Moverare-Skrtic, S. et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat. Med. 20, 1279–1288 (2014).
Johnson, M. L. LRP5 and bone mass regulation: where are we now? BoneKEy Rep. 1, 1 (2012).
van Dijk, F. S. et al. PLS3 mutations in X-linked osteoporosis with fractures. N. Engl. J. Med. 369, 1529–1536 (2013).
Laine, C. M. et al. A novel splice mutation in PLS3 causes X-linked early onset low-turnover osteoporosis. J. Bone Miner. Res. 30, 510–518 (2015).
Munns, C. F. et al. Homozygosity for frameshift mutations in XYLT2 result in a spondylo-ocular syndrome with bone fragility, cataracts, and hearing defects. Am. J. Hum. Genet. 96, 971–978 (2015).
Rutsch, F. et al. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am. J. Hum. Genet.96, 275–282 (2015).
Jang, M. A. et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am. J. Hum. Genet.96, 266–274 (2015).
Kiel, D. P. et al. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med. Genet. 8 (Suppl. 1), S14 (2007).
Richards, J. B. et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371, 1505–1512 (2008).
Styrkarsdottir, U. et al. Multiple genetic loci for bone mineral density and fractures. N. Engl. J. Med. 358, 2355–2365 (2008).
Rivadeneira, F. et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 41, 1199–1206 (2009).
Timpson, N. J. et al. Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum. Mol. Genet. 18, 1510–1517 (2009).
Cho, Y. S. et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 41, 527–534 (2009).
Kung, A. W. et al. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am. J. Hum. Genet. 86, 229–239 (2010).
Duncan, E. L. et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 7, e1001372 (2011).
Farber, C. R. Systems-level analysis of genome-wide association data. G3 (Bethesda) 3, 119–129 (2013).
He, H. et al. Integrative analysis of GWASs, human protein interaction, and gene expression identified gene modules associated with BMDs. J. Clin. Endocrinol. Metab. 99, E2392–E2399 (2014).
Kryukov, G. V., Shpunt, A., Stamatoyannopoulos, J. A. & Sunyaev, S. R. Power of deep, all-exon resequencing for discovery of human trait genes. Proc. Natl Acad. Sci. USA 106, 3871–3876 (2009).
Tennessen, J. A. et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 337, 64–69 (2012).
Auer, P. L. & Lettre, G. Rare variant association studies: considerations, challenges and opportunities. Genome Med. 7, 16 (2015).
Styrkarsdottir, U. et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 497, 517–520 (2013).
Zheng, H. et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 526, 112–117 (2015).
Zhang, L. et al. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum. Mol. Genet. 23, 1923–1933 (2014).
Kiel, D. et al. Targeted sequencing of previously identified loci associated with BMD [abstract #SU0158]. J. Bone Miner. Res. 27 (Suppl. 1), S261 (2012).
Johnsson, M., Jonsson, K. B., Andersson, L., Jensen, P. & Wright, D. Genetic regulation of bone metabolism in the chicken: similarities and differences to mammalian systems. PLoS Genet. 11, e1005250 (2015).
Ackert-Bicknell, C. L. et al. Mouse BMD quantitative trait loci show improved concordance with human genome-wide association loci when recalculated on a new, common mouse genetic map. J. Bone Miner. Res. 25, 1808–1820 (2010).
Wein, M. N. et al. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J. Bone Miner. Res. 30, 400–411 (2015).
Johnson, M. L. Unlocking the sost gene. J. Bone Miner. Res. 30, 397–399 (2015).
Kramer, I., Baertschi, S., Halleux, C., Keller, H. & Kneissel, M. Mef2c deletion in osteocytes results in increased bone mass. J. Bone Miner. Res. 27, 360–373 (2012).
Johnson, M. E. et al. A ChIP-seq-defined genome-wide map of MEF2C binding reveals inflammatory pathways associated with its role in bone density determination. Calcif. Tissue Int. 94, 396–402 (2014).
Kenney-Hunt, J. P. et al. Quantitative trait loci for body size components in mice. Mamm. Genome 17, 526–537 (2006).
Marchini, M. et al. Impacts of genetic correlation on the independent evolution of body mass and skeletal size in mammals. BMC Evol. Biol. 14, 258 (2014).
Lagerholm, S. et al. Identification of candidate gene regions in the rat by co-localization of QTLs for bone density, size, structure and strength. PLoS ONE 6, e22462 (2011).
Nikolskiy, I. et al. Using whole-genome sequences of the LG/J and SM/J inbred mouse strains to prioritize quantitative trait genes and nucleotides. BMC Genomics 16, 415 (2015).
Austin, C. P. et al. The Knockout Mouse Project. Nat. Genet. 36, 921–924 (2004).
Auwerx, J. et al. The European dimension for the mouse genome mutagenesis program. Nat. Genet. 36, 925–927 (2004).
The International Mouse Knockout Consortium. A mouse for all reasons. Cell 128, 9–13 (2007).
Ringwald, M. et al. The IKMC web portal: a central point of entry to data and resources from the International Knockout Mouse Consortium. Nucleic Acids Res. 39, D849–D855 (2011).
de Vrieze, E. et al. Prednisolone induces osteoporosis-like phenotype in regenerating zebrafish scales. Osteoporos. Int. 25, 567–578 (2014).
Mackay, E. W., Apschner, A. & Schulte-Merker, S. A bone to pick with zebrafish. BoneKEy Rep. 2, 445 (2013).
Gebruers, E. et al. A phenotypic screen in zebrafish identifies a novel small-molecule inducer of ectopic tail formation suggestive of alterations in non-canonical Wnt/PCP signaling. PLoS ONE 8, e83293 (2013).
de Vrieze, E., Zethof, J., Schulte-Merker, S., Flik, G. & Metz, J. R. Identification of novel osteogenic compounds by an ex-vivo sp7:luciferase zebrafish scale assay. Bone 74, 106–113 (2015).
Zhang, L. C. et al. A genome-wide association study of limb bone length using a Large White × Minzhu intercross population. Genet. Sel. Evol. 46, 56 (2014).
Zhu, J. et al. A systems genetics study of swine illustrates mechanisms underlying human phenotypic traits. BMC Genomics 16, 88 (2015).
Saatchi, M., Schnabel, R. D., Taylor, J. F. & Garrick, D. J. Large-effect pleiotropic or closely linked QTL segregate within and across ten US cattle breeds. BMC Genomics 15, 442 (2014).
Sivakumaran, S. et al. Abundant pleiotropy in human complex diseases and traits. Am. J. Hum. Genet. 89, 607–618 (2011).
Wang, Y. et al. Pleiotropy analysis of quantitative traits at gene level by multivariate functional linear models. Genet. Epidemiol. 39, 259–275 (2015).
Albagha, O. M. et al. Genome-wide association identifies three new susceptibility loci for Paget's disease of bone. Nat. Genet. 43, 685–689 (2011).
Karasik, D. et al. Genome-wide pleiotropy of osteoporosis-related phenotypes: the Framingham Study. J. Bone Miner. Res. 25, 1555–1563 (2010).
Lee, S. H., Yang, J., Goddard, M. E., Visscher, P. M. & Wray, N. R. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics 28, 2540–2542 (2012).
Smoller, J. W. et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381, 1371–1379 (2013).
Sun, X. et al. Genetic and environmental correlations between bone geometric parameters and body compositions. Calcif. Tissue Int. 79, 43–49 (2006).
Moseley, K. F., Dobrosielski, D. A., Stewart, K. J., Sellmeyer, D. E. & Jan De Beur, S. M. Lean mass predicts hip geometry in men and women with non-insulin-requiring type 2 diabetes mellitus. J. Clin.Densitom. 14, 332–339 (2011).
Deng, F. Y. et al. Bivariate whole genome linkage analysis for femoral neck geometric parameters and total body lean mass. J. Bone Miner. Res. 22, 808–816 (2007).
Karasik, D. et al. Bivariate genome-wide linkage analysis of femoral bone traits and leg lean mass: Framingham study. J. Bone Miner. Res. 24, 710–718 (2009).
Gupta, M. et al. Identification of homogeneous genetic architecture of multiple genetically correlated traits by block clustering of genome-wide associations. J. Bone Miner. Res. 26, 1261–1271 (2011).
Sun, L. et al. Bivariate genome-wide association analyses of femoral neck bone geometry and appendicular lean mass. PLoS ONE 6, e27325 (2011).
Guo, Y. F. et al. Suggestion of GLYAT gene underlying variation of bone size and body lean mass as revealed by a bivariate genome-wide association study. Hum. Genet. 132, 189–199 (2013).
Brotto, M. & Johnson, M. Endocrine crosstalk between muscle and bone. Curr. Osteoporos. Rep. 12, 135–141 (2014).
Kernstock, S. et al. Lysine methylation of VCP by a member of a novel human protein methyltransferase family. Nat. Commun. 3, 1038 (2012).
Cloutier, P., Lavallee-Adam, M., Faubert, D., Blanchette, M. & Coulombe, B. A newly uncovered group of distantly related lysine methyltransferases preferentially interact with molecular chaperones to regulate their activity. PLoS Genet. 9, e1003210 (2013).
Karasik, D. How pleiotropic genetics of the musculoskeletal system can inform genomics and phenomics of aging. Age (Dordr.) 33, 49–62 (2011).
Cauley, J. A. et al. Successful skeletal aging: a marker of low fracture risk and longevity. The Study of Osteoporotic Fractures (SOF). J. Bone Miner. Res. 24, 134–143 (2009).
Brennan, T. A. et al. Mouse models of telomere dysfunction phenocopy skeletal changes found in human age-related osteoporosis. Dis. Model. Mech. 7, 583–592 (2014).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Gilson, H. et al. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148, 452–460 (2007).
Bialek, P. et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone 60, 162–171 (2014).
Mendias, C. L. et al. Haploinsufficiency of myostatin protects against aging-related declines in muscle function and enhances the longevity of mice. Aging Cell 14, 704–706 (2015).
Tagliaferri, C., Wittrant, Y., Davicco, M.-J., Walrand, S. & Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 21, 55–70 (2015).
Karasik, D. & Zinder, M. The genetic pleiotropy of musculoskeletal aging. Front. Physiol. 3, 303 (2012).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Hartley, S. W., Monti, S., Liu, C.-T., Steinberg, M. H. & Sebastiani, P. Bayesian methods for multivariate modeling of pleiotropic SNP associations and genetic risk prediction. Front. Genet. 3, 176 (2012).
Cushman, S. A. Grand challenges in evolutionary and population genetics: the importance of integrating epigenetics, genomics, modeling, and experimentation. Front. Genet. 5, 197 (2014).
Savage, N. Bioinformatics: big data versus the big C. Nature 509, S66–67 (2014).
Bjornerem, A. et al. Genetic and environmental variances of bone microarchitecture and bone remodeling markers: a twin study. J. Bone Miner. Res. 30, 519–527 (2015).
Drissi, H., Paglia, D. N., Alaee, F. & Yoshida, R. Constructing the toolbox: patient-specific genetic factors of altered fracture healing. Genes Dis. 1, 140–148 (2014).
Fenger, M. Next generation genetics. Front. Genet. 5, 322 (2014).
Richards, J. B., Zheng, H. F. & Spector, T. D. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat. Rev. Genet. 13, 576–588 (2012).
Clark, G. R. & Duncan, E. L. The genetics of osteoporosis. Br. Med. Bull. 113, 73–81 (2015).
Eriksson, J. et al. Limited clinical utility of a genetic risk score for the prediction of fracture risk in elderly subjects. J. Bone Miner. Res. 30, 184–194 (2015).
Tran, B. H., Center, J. R. & Nguyen, N. T. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed Rosen, C. J.) 376–388 (Wiley-Blackwell, 2013).
Collins, F. S. et al. New goals for the U. S. Human Genome Project: 1998–2003. Science 282, 682–689 (1998).
Hsu, Y. H. et al. An integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility loci for osteoporosis-related traits. PLoS Genet. 6, e1000977 (2010).
Hsu, Y. H. & Kiel, D. P. Clinical review: genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed. J. Clin. Endocrinol. Metab. 97, E1958–E1977 (2012).
Xiao, S. M. et al. Post-genome wide association studies and functional analyses identify association of MPP7 gene variants with site-specific bone mineral density. Hum. Mol. Genet. 21, 1648–1657 (2012).
Sims, A. M. et al. Genetic analyses in a sample of individuals with high or low BMD shows association with multiple Wnt pathway genes. J. Bone Miner. Res. 23, 499–506 (2008).
Wang, J. et al. Exome sequencing reveals a novel PTHLH mutation in a Chinese pedigree with brachydactyly type E and short stature. Clin. Chim. Acta 446, 9–14 (2015).
Chesi, A. et al. A trans-ethnic genome-wide association study identifies gender-specific loci influencing pediatric aBMD and BMC at the distal radius. Hum. Mol. Genet. 24, 5053–5059 (2015).
Garcia-Ibarbia, C. et al. Contribution of genetic and epigenetic mechanisms to Wnt pathway activity in prevalent skeletal disorders. Gene 532, 165–172 (2013).
Guo, Y. et al. IL21R and PTH may underlie variation of femoral neck bone mineral density as revealed by a genome-wide association study. J. Bone Miner. Res. 25, 1042–1048 (2010).
Sobacchi, C., Schulz, A., Coxon, F. P., Villa, A. & Helfrich, M. H. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 9, 522–536 (2013).
Duncan, E. L. et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 7, e1001372 (2011).
Toka, H. R. New functional aspects of the extracellular calcium-sensing receptor. Curr. Opin. Nephrol. Hypertens. 23, 352–360 (2014).
Masi, L. et al. Taxonomy of rare genetic metabolic bone disorders. Osteoporos. Int. 26, 2529–2558 (2015).
Jin, W. et al. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J. Clin. Invest. 118, 1858–1866 (2008).
Richards, J. B. et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann. Intern. Med. 151, 528–537 (2009).
Paternoster, L. et al. Genetic determinants of trabecular and cortical volumetric bone mineral densities and bone microstructure. PLoS Genet. 9, e1003247 (2013).
Smith, E. P. et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 331, 1056–1061 (1994).
Jaeger, M. et al. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur J. Clin. Microbiol. Infect. Dis. 34, 963–974 (2015).
Kung, A. W. C. et al. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am. J. Hum. Genet. 86, 229–239 (2010).
Zanotti, S. & Canalis, E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif. Tissue Int. 90, 69–75 (2012).
Li, L. et al. JAG1 mutation spectrum and origin in chinese children with clinical features of alagille syndrome. PLoS ONE 10, e0130355 (2015).
Xiong, L. et al. Lrp4 in osteoblasts suppresses bone formation and promotes osteoclastogenesis and bone resorption. Proc. Natl Acad. Sci. USA 112, 3487–3492 (2015).
Leupin, O. et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J. Biol. Chem. 286, 19489–19500 (2011).
Zheng, H. F. et al. Meta-analysis of genome-wide studies identifies MEF2C SNPs associated with bone mineral density at forearm. J. Med. Genet. 50, 473–478 (2013).
Fahiminiya, S. et al. Osteoporosis caused by mutations in PLS3: clinical and bone tissue characteristics. J. Bone Miner. Res. 29, 1805–1814 (2014).
Nissenson, R. A. & Juppner, H. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed Rosen, C. J.) 208–214 (Wiley-Blackwell,2013).
Wysolmerski, J. J. Parathyroid Hormone-Related Protein (Wiley-Blackwell, 2013).
Komori, T. et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997).
Otto, F. et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–7671 (1997).
Mundlos, S. et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89, 773–779 (1997).
Liu, Y. J., Zhang, L., Papasian, C. J. & Deng, H. W. Genome-wide association studies for osteoporosis: a 2013 update. J. Bone Metab. 21, 99–116 (2014).
Vannahme, C. et al. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J. Biol. Chem. 277, 37977–37986 (2002).
Lapunzina, P. et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am. J. Hum. Genet. 87, 110–114 (2010).
Wu, Y. F., Matsuo, N., Sumiyoshi, H. & Yoshioka, H. Sp7/Osterix is involved in the up-regulation of the mouse pro-α1(V) collagen gene (Col5a1) in osteoblastic cells. Matrix Biol. 29, 701–706 (2010).
Lefebvre, V. & de Crombrugghe, B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 16, 529–40 (1998).
Ralston, S. H. & Albagha, O. M. Genetics of Paget's disease of bone. Curr. Osteoporos. Rep. 12, 263–271 (2014).
Sobacchi, C. et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 39, 960–962 (2007).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013).
Yu, B. et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB. Nat. Med. 20, 1009–1017 (2014).
Kou, I. et al. Common variants in a novel gene, FONG on chromosome 2q33.1 confer risk of osteoporosis in Japanese. PLoS ONE 6, e19641 (2011).
Niu, T. et al. Identification of IDUA and WNT16 phosphorylation-related non-synonymous polymorphisms for bone mineral density in meta-analyses of genome-wide association studies. J. Bone Miner. Res. 31, 358–368 (2016).
Cheung, C. L., Chan, V. & Kung, A. W. A differential association of ALOX15 polymorphisms with bone mineral density in pre- and post-menopausal women. Hum. Hered. 65, 1–8 (2008).
Acknowledgements
F.R.'s research is supported by a grant from the Netherlands Organization for Scientific Research (NWO-ZonMw VIDI 016.136.367). D.K. is supported by ERC FP7-PEOPLE-2012-Marie Curie Career Integration Grants and Israel Science Foundation (No. 1283/14). M.L.J.'s work is supported by a grant from the NIH (NIA P01 AG039355). The original artwork for the skeleton drawing in Fig. 2 is from a collection of anatomical drawings by the late Cecil G. Johnson (1920–2004), uncle of M.L.J.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and made substantial contribution to discussion of content, writing and reviewing/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Osteoporosis-related loci discovered by GWAS, for BMD, bone ultrasound, and fracture, with the nearest candidate genes. (PDF 311 kb)
Rights and permissions
About this article
Cite this article
Karasik, D., Rivadeneira, F. & Johnson, M. The genetics of bone mass and susceptibility to bone diseases. Nat Rev Rheumatol 12, 323–334 (2016). https://doi.org/10.1038/nrrheum.2016.48
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.48
This article is cited by
-
Feature selection approaches identify potential plasma metabolites in postmenopausal osteoporosis patients
Metabolomics (2022)
-
Postmenopausal Osteoporosis reference genes for qPCR expression assays
Scientific Reports (2019)
-
Impaired Wnt Signaling in the Prefrontal Cortex of Alzheimer’s Disease
Molecular Neurobiology (2019)
-
WNT3A accelerates delayed alveolar bone repair in ovariectomized mice
Osteoporosis International (2019)
-
Identification of pleiotropic genetic variants affecting osteoporosis risk in a Korean elderly cohort
Journal of Bone and Mineral Metabolism (2019)